| << Chapter < Page | Chapter >> Page > |
A 0.500 kg aluminum pan on a stove is used to heat 0.250 liters of water from to . (a) How much heat is required? What percentage of the heat is used to raise the temperature of (b) the pan and (c) the water?
Strategy
The pan and the water are always at the same temperature. When you put the pan on the stove, the temperature of the water and the pan is increased by the same amount. We use the equation for the heat transfer for the given temperature change and mass of water and aluminum. The specific heat values for water and aluminum are given in [link] .
Solution
Because water is in thermal contact with the aluminum, the pan and the water are at the same temperature.
Thus, the amount of heat going into heating the pan is
and the amount going into heating the water is
Discussion
In this example, the heat transferred to the container is a significant fraction of the total transferred heat. Although the mass of the pan is twice that of the water, the specific heat of water is over four times greater than that of aluminum. Therefore, it takes a bit more than twice the heat to achieve the given temperature change for the water as compared to the aluminum pan.
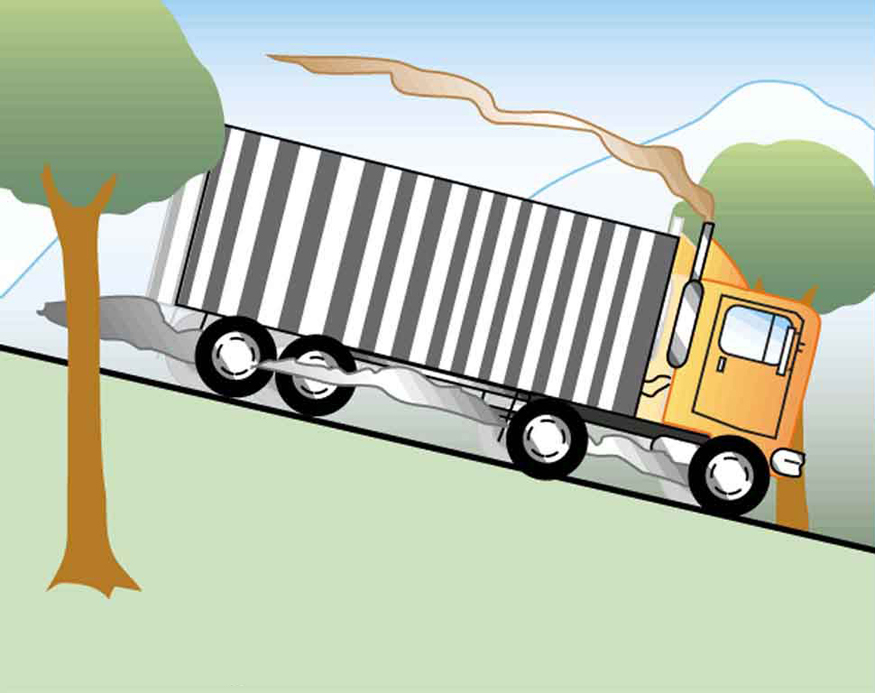
Truck brakes used to control speed on a downhill run do work, converting gravitational potential energy into increased internal energy (higher temperature) of the brake material. This conversion prevents the gravitational potential energy from being converted into kinetic energy of the truck. The problem is that the mass of the truck is large compared with that of the brake material absorbing the energy, and the temperature increase may occur too fast for sufficient heat to transfer from the brakes to the environment.
Calculate the temperature increase of 100 kg of brake material with an average specific heat of if the material retains 10% of the energy from a 10,000-kg truck descending 75.0 m (in vertical displacement) at a constant speed.
Strategy
If the brakes are not applied, gravitational potential energy is converted into kinetic energy. When brakes are applied, gravitational potential energy is converted into internal energy of the brake material. We first calculate the gravitational potential energy that the entire truck loses in its descent and then find the temperature increase produced in the brake material alone.

Notification Switch
Would you like to follow the 'College physics' conversation and receive update notifications?