| << Chapter < Page | Chapter >> Page > |
If the reaction is allowed to get too hot then several possible side reactions can occur. In THF reaction with the solvent occurs:
Alternatively, a transition metal catalyzed radical coupling between the Grignard and unreacted alkyl halide is observed irrespective of the identity of the solvent, [link] .
The mechanism for Grignard formation is thought to be radical in nature; however, a study of the surface of the magnesium during the reaction has shown the presence of corrosion pits. It is generally agreed that initiation occurs at surface dislocations, but the major reaction occurs at a polished surface.
The kinetics of the reaction is 1 st order with respect to the alkyl halide concentration, but it has also been claimed to be 1 st order with respect to the solvent concentration. It has therefore been concluded that the rate-determining step involves the metal solvent interface.
The reaction of magnesium with aryl bromides has been studied and is proposed to occur by two reactions. The first involves electron transfer between the aryl halide and the metal, while the second involves aryl radical formation.
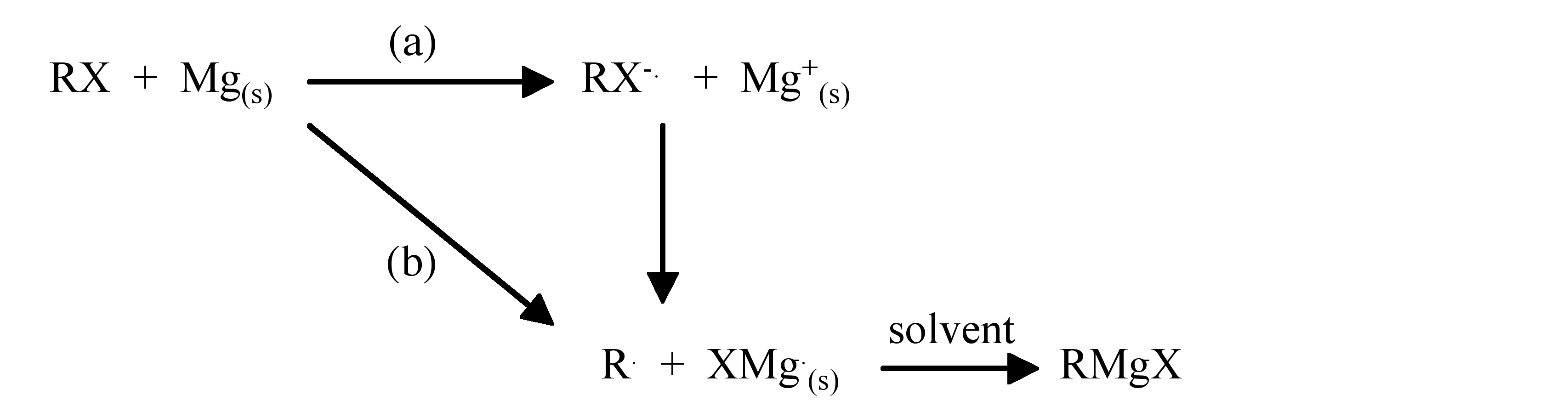
A number of alternative synthetic routes are used with polyhalogenated hydrocarbons, [link] and [link] , and where the alkyl radical is unstable, [link] .
The solid state structure of Grignard reagents is controlled by the presence and identity of the solvent used in the synthesis. In this regard the size and the basicity of the solvent is important. For example, the structure of EtMgBr crystallized from diethyl ether exists as a 4-ccordinate monomer ( [link] a), while the use of the sterically less demanding THF results in a 5-coordinate monomeric structure ( [link] b). In contrast, the use of triethylamine yields a dimeric bromide bridged structure ( [link] c), and the use of a chelate bidentate amine gives a structure ( [link] d) similar to that observed with diethyl ether ( [link] a).

In solution, Grignards are fluxional such that no single defined structure is present. The series of exchange reactions are known as an extended Schlenk equilibrium ( [link] ).

It is observed that Grignard solutions are also slightly conducting, and magnesium is deposited at both the anode and cathode suggesting the formation of RMg + and [RMgX 2 ] - . The alkyl/halide exchange is thought to occur through a bridging intermediate ( [link] ).

Dialkyl magnesium compounds are involatile white solids. They generally have similar reactivity to their Grignard analogs.
The most common synthesis of R 2 Mg is by the reaction of a Grignard with dioxane (C 4 H 8 O 2 ), [link] , where the precipitation of the dihalide is the reaction driving force.
This method is useful for the synthesis of cyclic compounds, [link] .

An alternative synthesis that does not require dioxane involves the metal exchange reaction between magnesium metal and a dialkyl mercury compound.

Notification Switch
Would you like to follow the 'Chemistry of the main group elements' conversation and receive update notifications?