| << Chapter < Page | Chapter >> Page > |
Nucleic acids are the most important macromolecules for the continuity of life. They carry the genetic blueprint of a cell and carry instructions for the functioning of the cell.
The two main types of nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) . DNA is the genetic material found in all living organisms, ranging from single-celled bacteria to multicellular mammals. It is found in the nucleus of eukaryotes and in the organelles, chloroplasts, and mitochondria. In prokaryotes, the DNA is not enclosed in a membranous envelope.
The entire genetic content of a cell is known as its genome, and the study of genomes is genomics. In eukaryotic cells but not in prokaryotes, DNA forms a complex with histone proteins to form chromatin, the substance of eukaryotic chromosomes. A chromosome may contain tens of thousands of genes. Many genes contain the information to make protein products; other genes code for RNA products. DNA controls all of the cellular activities by turning the genes “on” or “off.”
The other type of nucleic acid, RNA, is mostly involved in protein synthesis. The DNA molecules never leave the nucleus but instead use an intermediary to communicate with the rest of the cell. This intermediary is the messenger RNA (mRNA) . Other types of RNA—like rRNA, tRNA, and microRNA—are involved in protein synthesis and its regulation.
DNA and RNA are made up of monomers known as nucleotides . The nucleotides combine with each other to form a polynucleotide , DNA or RNA. Each nucleotide is made up of three components: a nitrogenous base, a pentose (five-carbon) sugar, and a phosphate group ( [link] ). Each nitrogenous base in a nucleotide is attached to a sugar molecule, which is attached to one or more phosphate groups.
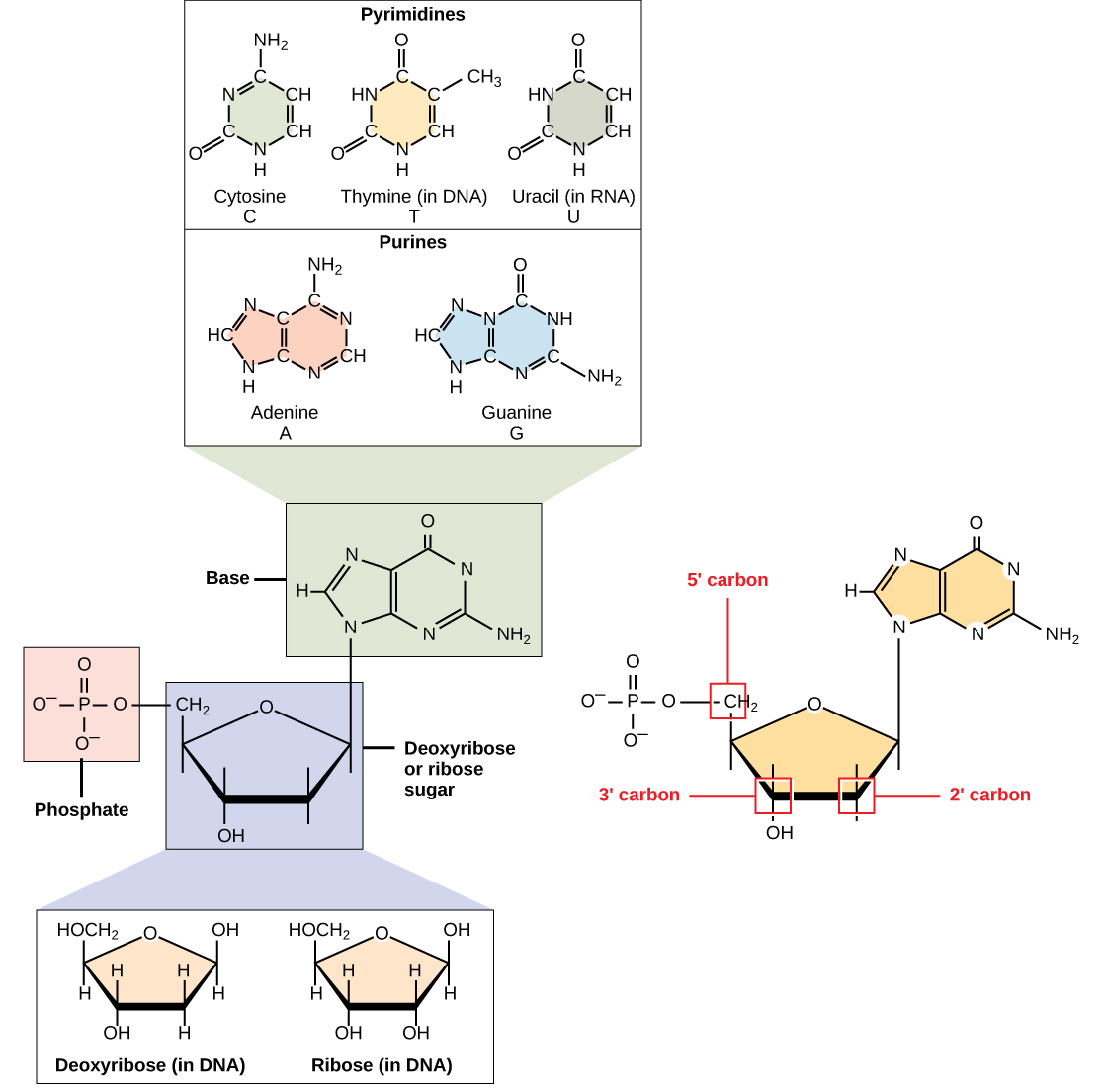
The nitrogenous bases, important components of nucleotides, are organic molecules and are so named because they contain carbon and nitrogen. They are bases because they contain an amino group that has the potential of binding an extra hydrogen, and thus, decreases the hydrogen ion concentration in its environment, making it more basic. Each nucleotide in DNA contains one of four possible nitrogenous bases: adenine (A), guanine (G) cytosine (C), and thymine (T).

Notification Switch
Would you like to follow the 'Biology' conversation and receive update notifications?