| << Chapter < Page | Chapter >> Page > |
We all experience fluctuations in our weight from time to time, but generally, most people’s weights fluctuate within a narrow margin, in the absence of extreme changes in diet and/or physical activity. This observation led some to propose a set-point theory of body weight regulation. The set-point theory asserts that each individual has an ideal body weight, or set point, which is resistant to change. This set-point is genetically predetermined and efforts to move our weight significantly from the set-point are resisted by compensatory changes in energy intake and/or expenditure (Speakman et al., 2011).
Some of the predictions generated from this particular theory have not received empirical support. For example, there are no changes in metabolic rate between individuals who had recently lost significant amounts of weight and a control group (Weinsier et al., 2000). In addition, the set-point theory fails to account for the influence of social and environmental factors in the regulation of body weight (Martin-Gronert&Ozanne, 2013; Speakman et al., 2011). Despite these limitations, set-point theory is still often used as a simple, intuitive explanation of how body weight is regulated.
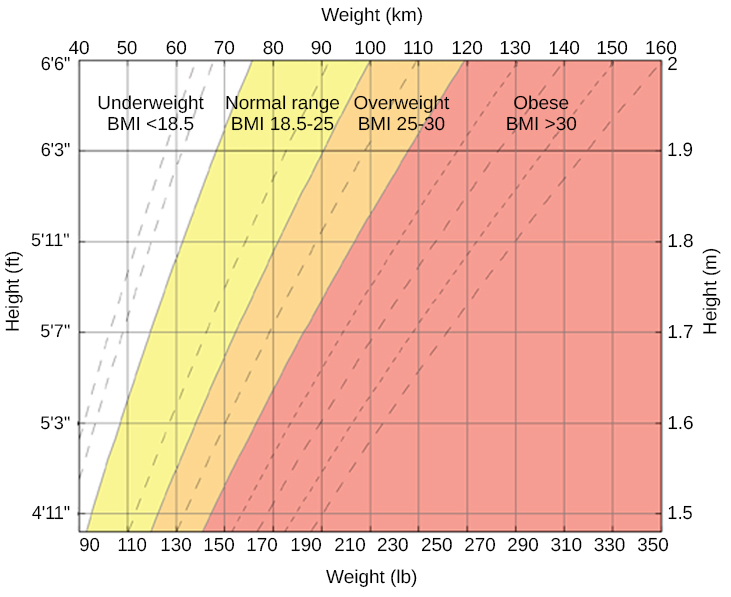
When someone weighs more than what is generally accepted as healthy for a given height, they are considered overweight or obese. According to the Centers for Disease Control and Prevention (CDC), an adult with a body mass index (BMI) between 25 and 29.9 is considered overweight ( [link] ). An adult with a BMI of 30 or higher is considered obese (Centers for Disease Control and Prevention [CDC], 2012). People who are so overweight that they are at risk for death are classified as morbidly obese. Morbid obesity is defined as having a BMI over 40. Note that although BMI has been used as a healthy weight indicator by the World Health Organization (WHO), the CDC, and other groups, its value as an assessment tool has been questioned. The BMI is most useful for studying populations, which is the work of these organizations. It is less useful in assessing an individual since height and weight measurements fail to account for important factors like fitness level. An athlete, for example, may have a high BMI because the tool doesn’t distinguish between the body’s percentage of fat and muscle in a person’s weight.
Being extremely overweight or obese is a risk factor for several negative health consequences. These include, but are not limited to, an increased risk for cardiovascular disease, stroke, Type 2 diabetes, liver disease, sleep apnea, colon cancer, breast cancer, infertility, and arthritis. Given that it is estimated that in the United States around one-third of the adult population is obese and that nearly two-thirds of adults and one in six children qualify as overweight (CDC, 2012), there is substantial interest in trying to understand how to combat this important public health concern.

Notification Switch
Would you like to follow the 'Psychology' conversation and receive update notifications?