| << Chapter < Page | Chapter >> Page > |
Does the uncharged conductor shown below experience a net electric force?
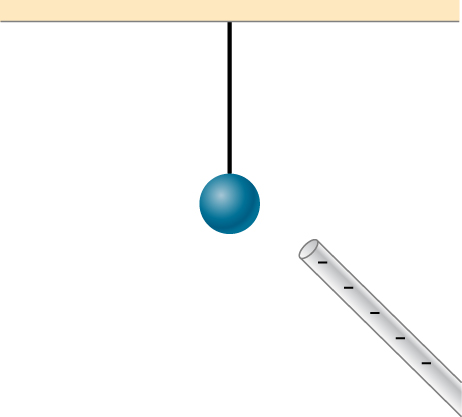
Yes, polarization charge is induced on the conductor so that the positive charge is nearest the charged rod, causing an attractive force.
While walking on a rug, a person frequently becomes charged because of the rubbing between his shoes and the rug. This charge then causes a spark and a slight shock when the person gets close to a metal object. Why are these shocks so much more common on a dry day?
Compare charging by conduction to charging by induction.
Charging by conduction is charging by contact where charge is transferred to the object. Charging by induction first involves producing a polarization charge in the object and then connecting a wire to ground to allow some of the charge to leave the object, leaving the object charged.
Small pieces of tissue are attracted to a charged comb. Soon after sticking to the comb, the pieces of tissue are repelled from it. Explain.
Trucks that carry gasoline often have chains dangling from their undercarriages and brushing the ground. Why?
This is so that any excess charge is transferred to the ground, keeping the gasoline receptacles neutral. If there is excess charge on the gasoline receptacle, a spark could ignite it.
Why do electrostatic experiments work so poorly in humid weather?
Why do some clothes cling together after being removed from the clothes dryer? Does this happen if they’re still damp?
The dryer charges the clothes. If they are damp, the presence of water molecules suppresses the charge.
Can induction be used to produce charge on an insulator?
Suppose someone tells you that rubbing quartz with cotton cloth produces a third kind of charge on the quartz. Describe what you might do to test this claim.
There are only two types of charge, attractive and repulsive. If you bring a charged object near the quartz, only one of these two effects will happen, proving there is not a third kind of charge.
A handheld copper rod does not acquire a charge when you rub it with a cloth. Explain why.
Suppose you place a charge q near a large metal plate. (a) If q is attracted to the plate, is the plate necessarily charged? (b) If q is repelled by the plate, is the plate necessarily charged?
a. No, since a polarization charge is induced. b. Yes, since the polarization charge would produce only an attractive force.
Suppose a speck of dust in an electrostatic precipitator has protons in it and has a net charge of −5.00 nC (a very large charge for a small speck). How many electrons does it have?
;
An amoeba has protons and a net charge of 0.300 pC. (a) How many fewer electrons are there than protons? (b) If you paired them up, what fraction of the protons would have no electrons?
A 50.0-g ball of copper has a net charge of . What fraction of the copper’s electrons has been removed? (Each copper atom has 29 protons, and copper has an atomic mass of 63.5.)
atomic mass of copper atom times
;
number of copper atoms
;
number of electrons equals 29 times number of atoms or
;
What net charge would you place on a 100-g piece of sulfur if you put an extra electron on 1 in of its atoms? (Sulfur has an atomic mass of 32.1 u.)
How many coulombs of positive charge are there in 4.00 kg of plutonium, given its atomic mass is 244 and that each plutonium atom has 94 protons?
;
;

Notification Switch
Would you like to follow the 'University physics volume 2' conversation and receive update notifications?