| << Chapter < Page | Chapter >> Page > |
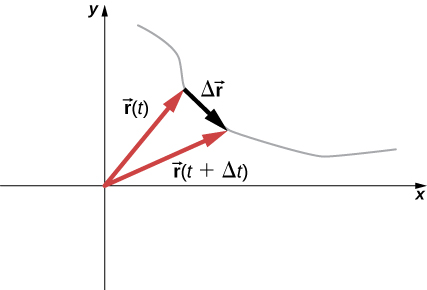
[link] can also be written in terms of the components of Since
we can write
where
If only the average velocity is of concern, we have the vector equivalent of the one-dimensional average velocity for two and three dimensions:
(a)
Speed
(b) From
[link] ,
Check Your Understanding The position function of a particle is (a) What is the instantaneous velocity at t = 3 s? (b) Is the average velocity between 2 s and 4 s equal to the instantaneous velocity at t = 3 s?
(a) Taking the derivative with respect to time of the position function, we have
(b) Since the velocity function is nonlinear, we suspect the average velocity is not equal to the instantaneous velocity. We check this and find
which is different from
When we look at the three-dimensional equations for position and velocity written in unit vector notation, [link] and [link] , we see the components of these equations are separate and unique functions of time that do not depend on one another. Motion along the x direction has no part of its motion along the y and z directions, and similarly for the other two coordinate axes. Thus, the motion of an object in two or three dimensions can be divided into separate, independent motions along the perpendicular axes of the coordinate system in which the motion takes place.
To illustrate this concept with respect to displacement, consider a woman walking from point A to point B in a city with square blocks. The woman taking the path from A to B may walk east for so many blocks and then north (two perpendicular directions) for another set of blocks to arrive at B . How far she walks east is affected only by her motion eastward. Similarly, how far she walks north is affected only by her motion northward.
In the kinematic description of motion, we are able to treat the horizontal and vertical components of motion separately. In many cases, motion in the horizontal direction does not affect motion in the vertical direction, and vice versa.

Notification Switch
Would you like to follow the 'University physics volume 1' conversation and receive update notifications?