| << Chapter < Page | Chapter >> Page > |
1. As in length, it is essential that we know when to use grams, kilograms and tons as units.
Work with a friend, taking turns. Take any three objects in your class. Hold each one separately and arrange them from the lightest to the heaviest. In which unit would you weigh these items: grams? kg?; ton?
Complete the table:
| Item | From light to heavy | Unit | |
| 1 . | .......................................... | .......................................... | .......................................... |
| 2 . | .......................................... | .......................................... | .......................................... |
| 3 . | .......................................... | .......................................... | .......................................... |
DO YOU STILL REMEMBER?
1 000 g = 1 kg
1 000 kg = 1 ton
Sometimes an object can be so light that you can’t weigh it on a normal scale. Tick the objects that you think weigh less than 1 gram.
| a feather | a rubber | a pin |
| a staple | an orange | a grain of sand |
| a hair elastic | a pen | a hair |
DID YOU KNOW?
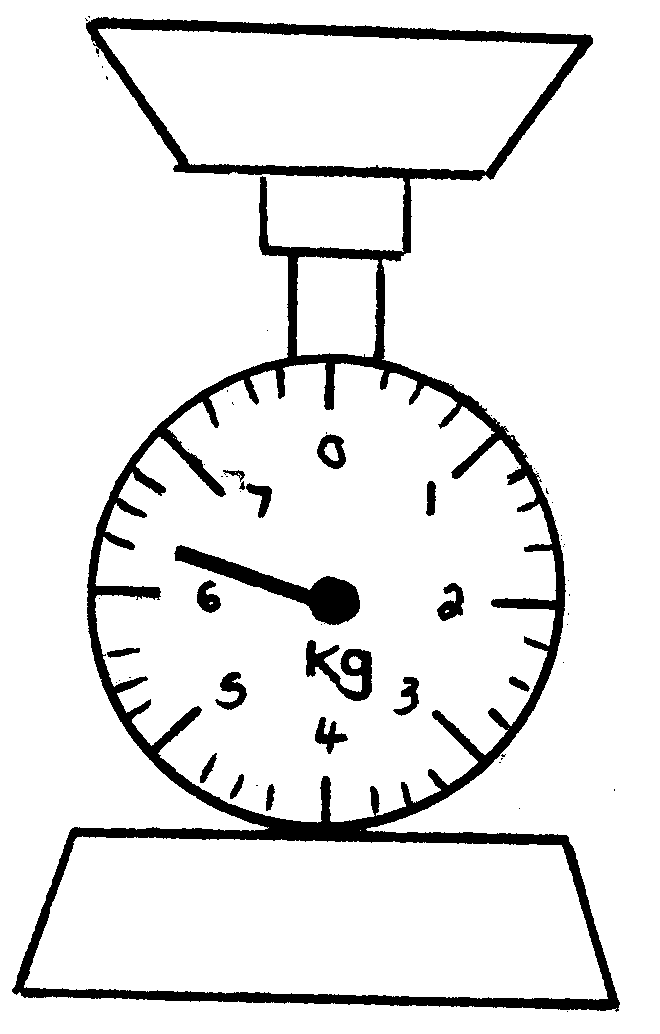
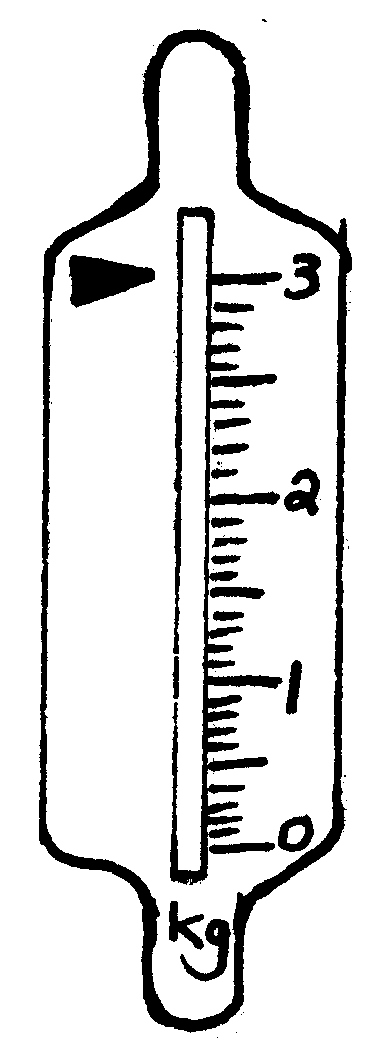
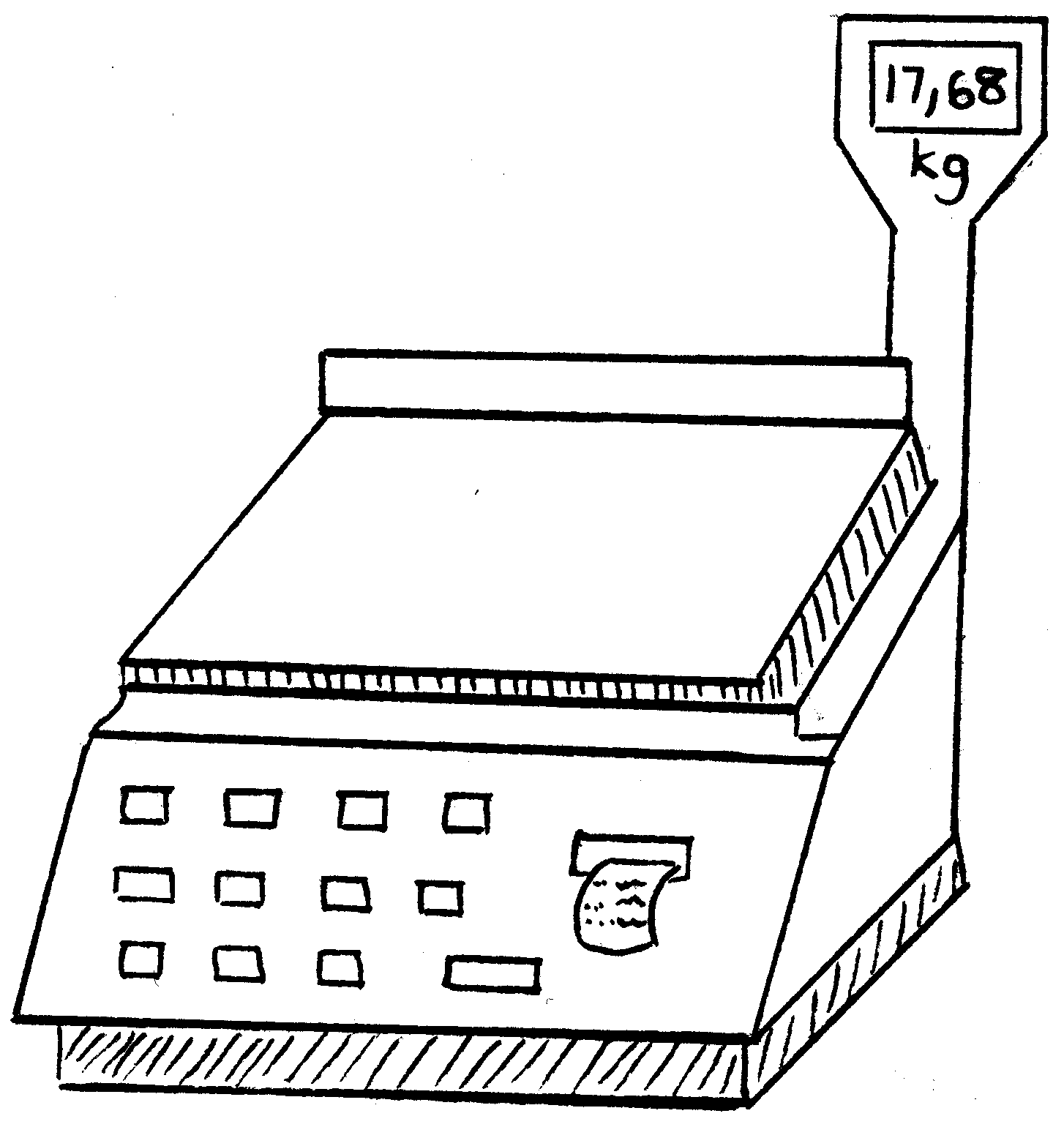
When we want to determine the mass of an object, it means that we want to know how heavy it is or how much it weighs . To be able to do this, we need a scale .
Please write down all the different kinds of scales you can think of:
1.1
1.2

1.3
1.4
1.5

1.6
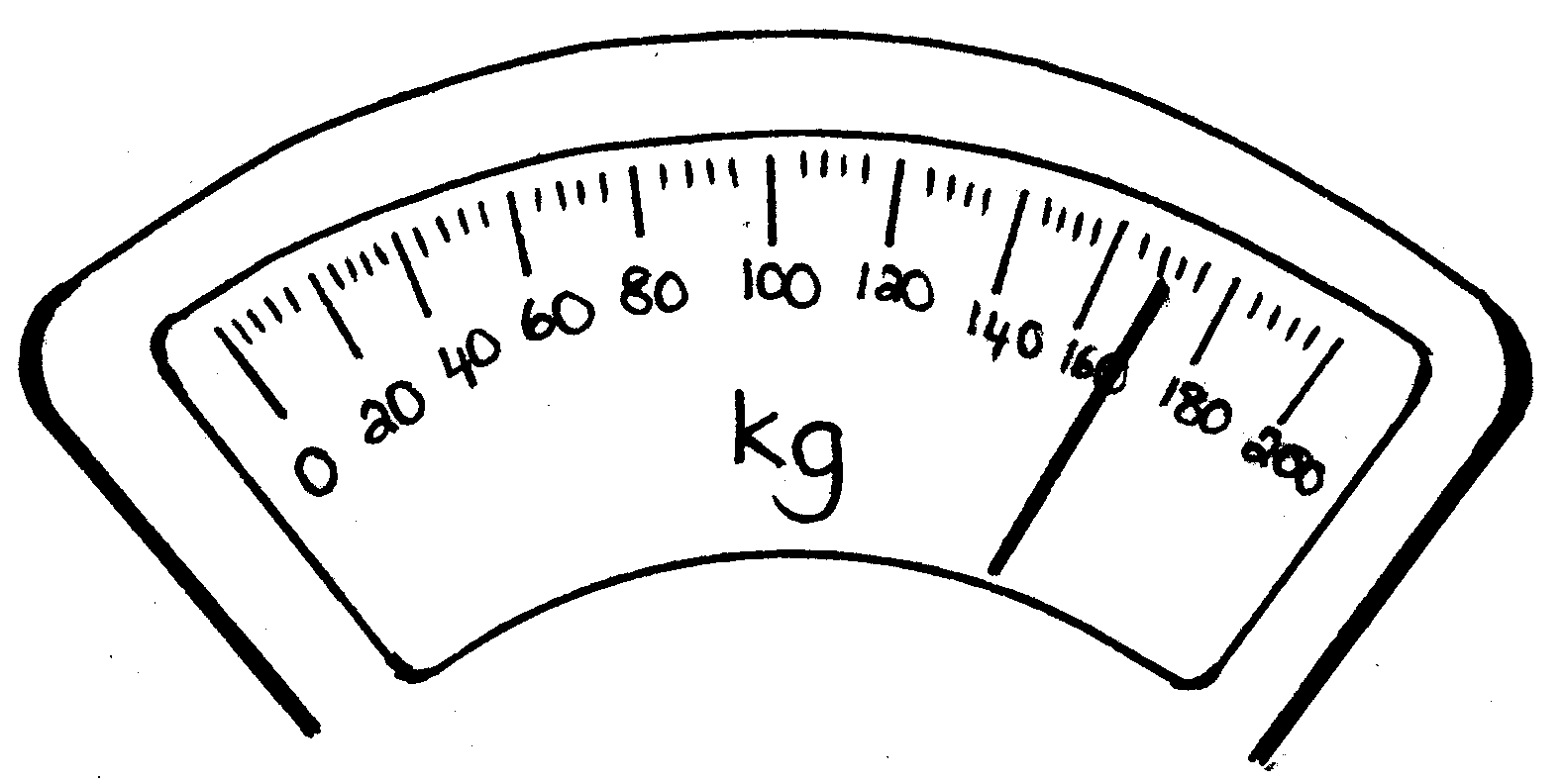
2. Kyk na die volgende tekeninge en skryf die massa van die voorwerpe neer.
2. Look at the following drawings and write down the mass of each object:
2.1

2.2

2.3
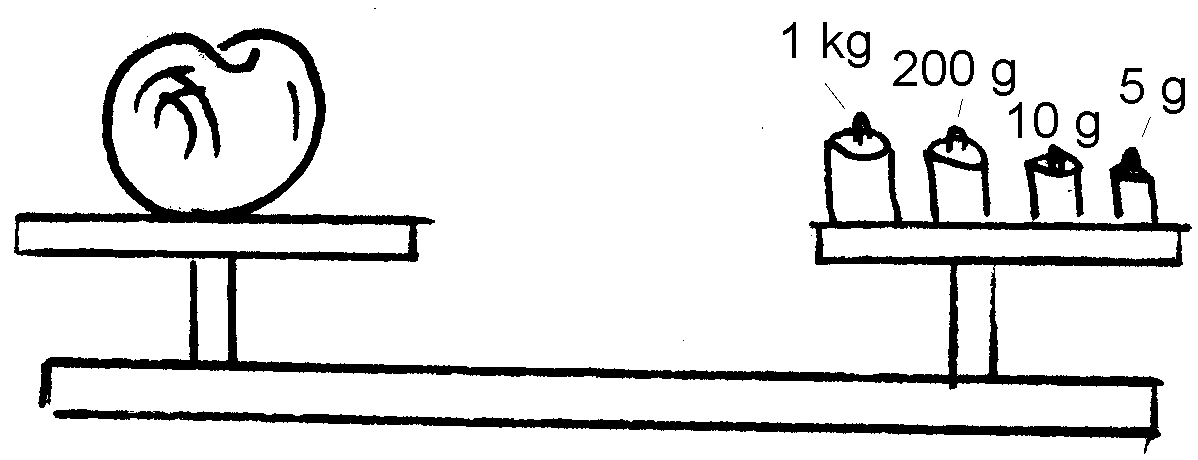
2.4
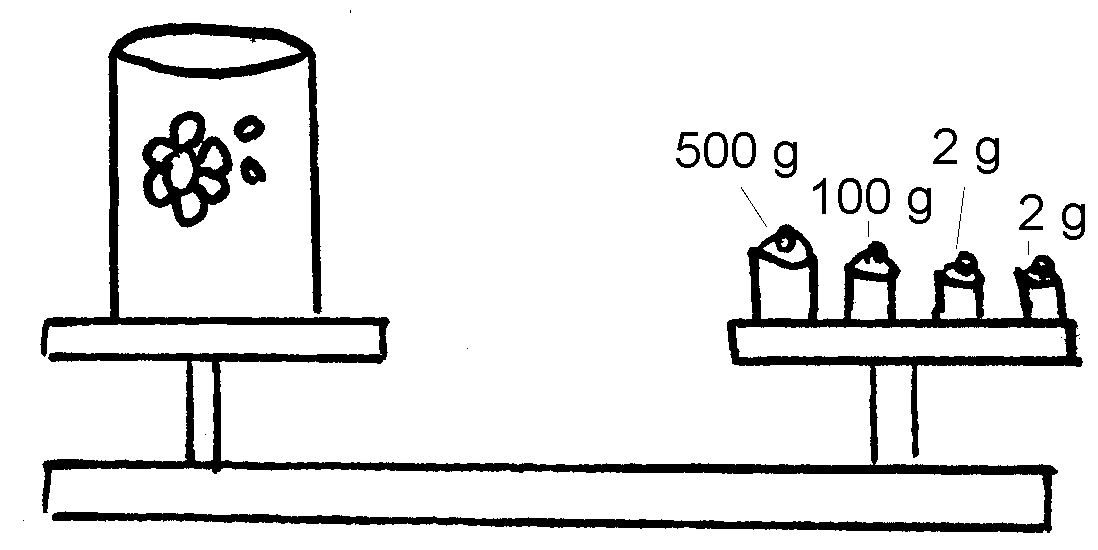
Work in groups of three. Bring the following items from home:
A scale; 1 cup of raw rice; 1 cup of sugar; 1 cup of flour; 1 cup of salt; 1 cup of all bran/corn flakes and 1 cup of raisins.(Remember to use the same cup each time you measure).
1. Determine the mass of each item and complete the table.
| Item | Mass in g estimated | Item weighed | Difference |
| .............................................. | ......................... | ..................... | ..................... |
| .............................................. | ......................... | ..................... | ..................... |
| .............................................. | ......................... | ..................... | ..................... |
| .............................................. | ......................... | ..................... | ..................... |
| .............................................. | ......................... | ..................... | ..................... |
| .............................................. | ......................... | ..................... | ..................... |
2. Which item is the heaviest?
3. Which item is the lightest?
LET US REVISE!

Notification Switch
Would you like to follow the 'Mathematics grade 5' conversation and receive update notifications?