| << Chapter < Page | Chapter >> Page > |
You will remember that when atoms bond, electrons are either shared or they are transferred between the atoms that are bonding. In covalent bonding, electrons are shared between the atoms. There is another type of bonding, where electrons are transferred from one atom to another. This is called ionic bonding .
Ionic bonding takes place when the difference in electronegativity between the two atoms is more than 1,7. This usually happens when a metal atom bonds with a non-metal atom. When the difference in electronegativity is large, one atom will attract the shared electron pair much more strongly than the other, causing electrons to be transferred from one atom to the other.
Example 1:
In the case of , the difference in electronegativity is 2,1. Sodium has only one valence electron, while chlorine has seven. Because the electronegativity of chlorine is higher than the electronegativity of sodium, chlorine will attract the valence electron of the sodium atom very strongly. This electron from sodium is transferred to chlorine. Sodium loses an electron and forms an ion. Chlorine gains an electron and forms an ion. The attractive force between the positive and negative ion holds the molecule together.
The balanced equation for the reaction is:
This can be represented using Lewis notation:
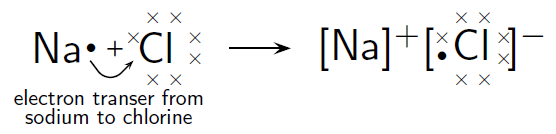
Example 2:
Another example of ionic bonding takes place between magnesium ( ) and oxygen ( ) to form magnesium oxide ( ). Magnesium has two valence electrons and an electronegativity of 1,2, while oxygen has six valence electrons and an electronegativity of 3,5. Since oxygen has a higher electronegativity, it attracts the two valence electrons from the magnesium atom and these electrons are transferred from the magnesium atom to the oxygen atom. Magnesium loses two electrons to form , and oxygen gains two electrons to form . The attractive force between the oppositely charged ions is what holds the molecule together.
The balanced equation for the reaction is:
Because oxygen is a diatomic molecule, two magnesium atoms will be needed to combine with one oxygen molecule (which has two oxygen atoms) to produce two molecules of magnesium oxide ( ).

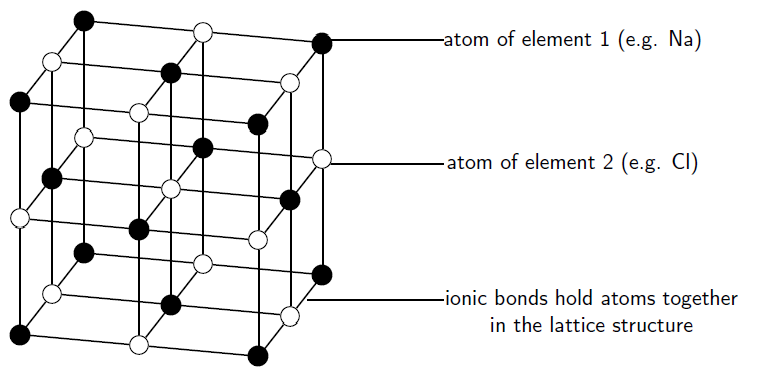
Ionic substances are actually a combination of lots of ions bonded together into a giant molecule. The arrangement of ions in a regular,geometric structure is called a crystal lattice . So in fact does not contain one and one ion, but rather a lot of these two ions arranged in a crystal lattice where the ratio of to ions is 1:1. The structure of a crystal lattice is shown in [link] .
Ionic compounds have a number of properties:

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 10 physical science [caps]' conversation and receive update notifications?