| << Chapter < Page | Chapter >> Page > |
Although we have used diagrams to show the structure of molecules, there are other forms of notation that can be used, such as Lewis notation and Couper notation . Lewis notation uses dots and crosses to represent the valence electrons on different atoms. The chemical symbol of the element is used to represent the nucleus and the core electrons of the atom.
So, for example, a hydrogen atom would be represented like this:

A chlorine atom would look like this:

A molecule of hydrogen chloride would be shown like this:

The dot and cross in between the two atoms, represent the pair of electrons that are shared in the covalent bond.
Represent the molecule using Lewis notation
The electron configuration of hydrogen is and the electron configuration for oxygen is . Each hydrogen atom has one valence electron, which is unpaired, and the oxygen atom has six valence electrons with two unpaired.

The water molecule is represented below.

Represent the molecule (hydrogen cyanide) using Lewis notation
The electron configuration of hydrogen is , the electron configuration of nitrogen is and for carbon is . This means that hydrogen has one valence electron which is unpaired, carbon has four valence electrons, all of which are unpaired, and nitrogen has five valence electrons, three of which are unpaired.
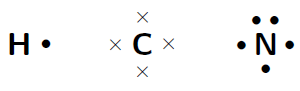
The molecule is represented below. Notice the three electron pairs between the nitrogen and carbon atom. Because these three covalent bonds are between the same two atoms, this is a triple bond.

Represent the molecule (hydrogen sulphide) using Lewis notation
Hydrogen has an electron configuration of and sulphur has an electron configuration of . Each hydrogen atom has one valence electron which is unpaired, and sulphur has six valence electrons. Although sulphur has a variable valency, we know that the sulphur will be able to form 2 bonds with the hydrogen atoms. In this case, the valency of sulphur must be two.

The molecule is represented below.

Another way of representing molecules is using Couper notation . In this case, only the electrons that are involved in the bond between the atoms are shown. A line is used for each covalent bond. Using Couper notation, a molecule of water and a molecule of would be represented as shown in figures [link] and [link] below.
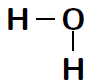
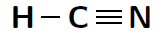


Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 10 physical science [caps]' conversation and receive update notifications?