| << Chapter < Page | Chapter >> Page > |
Aim:
To demonstrate that a homogeneous salt solution can be separated using physical methods.
Apparatus:
glass beaker, salt, water, retort stand, bunsen burner.
Method:
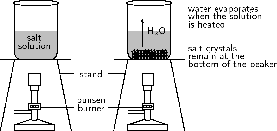
Results:
The water evaporates from the beaker and tiny grains of salt remain at the bottom. (You may also observe grains of salt on the walls of the beaker.)
Conclusion:
The salt solution, which is a homogeneous mixture of salt and water, has been separated using heating and evaporation.
Work in groups of 3-4
Imagine that you have been given a container which holds a mixture of sand, iron filings (small pieces of iron metal), salt and small stones of different sizes. Is this a homogeneous or a heterogeneous mixture? In your group, discuss how you would go about separating this mixture into the four materials that it contains.
Any material that is not a mixture, is called a pure substance . Pure substances include elements and compounds . It is much more difficult to break down pure substances into their parts, and complex chemical methods are needed to do this.
An element is a chemical substance that can't be divided or changed into other chemical substances by any ordinary chemical means. The smallest unit of an element is the atom .
An element is a substance that cannot be broken down into other substances through chemical means.
There are 112 officially named elements and about 118 known elements. Most of these are natural, but some are man-made. The elements we know are represented in the Periodic Table of the Elements , where each element is abbreviated to a chemical symbol . Examples of elements are magnesium (Mg), hydrogen (H), oxygen (O) and carbon (C). On the Periodic Table you will notice that some of the abbreviations do not seem to match the elements they represent. The element iron, for example, has the chemical formula Fe. This is because the elements were originally given Latin names. Iron has the abbreviation Fe because its Latin name is 'ferrum'. In the same way, sodium's Latin name is 'natrium' (Na) and gold's is 'aurum' (Au).
A compound is a chemical substance that forms when two or more elements combine in a fixed ratio. Water (H 2 O), for example, is a compound that is made up of two hydrogen atoms for every one oxygen atom. Sodium chloride (NaCl) is a compound made up of one sodium atom for every chlorine atom. An important characteristic of a compound is that it has a chemical formula , which describes the ratio in which the atoms of each element in the compound occur.
A substance made up of two or more elements that are joined together in a fixed ratio.
[link] might help you to understand the difference between the terms element , mixture and compound . Iron (Fe) and sulphur (S) are two elements. When they are added together, they form a mixture of iron and sulphur. The iron and sulphur are not joined together. However, if the mixture is heated, a new compound is formed, which is called iron sulphide (FeS). In this compound, the iron and sulphur are joined to each other in a ratio of 1:1. In other words, one atom of iron is joined to one atom of sulphur in the compound iron sulphide.

| Substance | Mixture or pure | Homogeneous or heterogeneous mixture |
| fizzy colddrink | ||
| steel | ||
| oxygen | ||
| iron filings | ||
| smoke | ||
| limestone (CaCO 3 ) |

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 10 physical science' conversation and receive update notifications?