| << Chapter < Page | Chapter >> Page > |
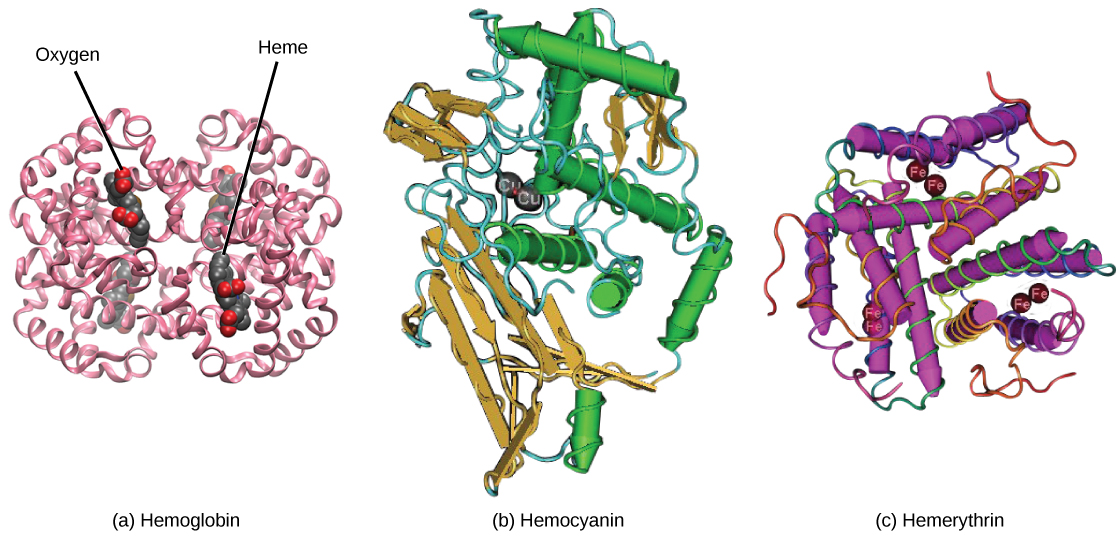
Not all organisms use hemoglobin as the method of oxygen transport. Invertebrates that utilize hemolymph rather than blood use different pigments to bind to the oxygen. These pigments use copper or iron to the oxygen. Invertebrates have a variety of other respiratory pigments. Hemocyanin, a blue-green, copper-containing protein, illustrated in [link] b is found in mollusks, crustaceans, and some of the arthropods. Chlorocruorin, a green-colored, iron-containing pigment is found in four families of polychaete tubeworms. Hemerythrin, a red, iron-containing protein is found in some polychaete worms and annelids and is illustrated in [link] c . Despite the name, hemerythrin does not contain a heme group and its oxygen-carrying capacity is poor compared to hemoglobin.
The small size and large surface area of red blood cells allows for rapid diffusion of oxygen and carbon dioxide across the plasma membrane. In the lungs, carbon dioxide is released and oxygen is taken in by the blood. In the tissues, oxygen is released from the blood and carbon dioxide is bound for transport back to the lungs. Studies have found that hemoglobin also binds nitrous oxide (NO). NO is a vasodilator that relaxes the blood vessels and capillaries and may help with gas exchange and the passage of red blood cells through narrow vessels. Nitroglycerin, a heart medication for angina and heart attacks, is converted to NO to help relax the blood vessels and increase oxygen flow through the body.
A characteristic of red blood cells is their glycolipid and glycoprotein coating; these are lipids and proteins that have carbohydrate molecules attached. In humans, the surface glycoproteins and glycolipids on red blood cells vary between individuals, producing the different blood types, such as A, B, and O. Red blood cells have an average life span of 120 days, at which time they are broken down and recycled in the liver and spleen by phagocytic macrophages, a type of white blood cell.
White blood cells, also called leukocytes (leuko = white), make up approximately one percent by volume of the cells in blood. The role of white blood cells is very different than that of red blood cells: they are primarily involved in the immune response to identify and target pathogens, such as invading bacteria, viruses, and other foreign organisms. White blood cells are formed continually; some only live for hours or days, but some live for years.

Notification Switch
Would you like to follow the 'Biology' conversation and receive update notifications?