| << Chapter < Page | Chapter >> Page > |
Although useful to explain the reactivity and chemical bonding of certain elements, the Bohr model of the atom does not accurately reflect how electrons are spatially distributed surrounding the nucleus. They do not circle the nucleus like the earth orbits the sun, but are found in electron orbitals . These relatively complex shapes result from the fact that electrons behave not just like particles, but also like waves. Mathematical equations from quantum mechanics known as wave functions can predict within a certain level of probability where an electron might be at any given time. The area where an electron is most likely to be found is called its orbital.
Recall that the Bohr model depicts an atom’s electron shell configuration. Within each electron shell are subshells, and each subshell has a specified number of orbitals containing electrons. While it is impossible to calculate exactly where an electron is located, scientists know that it is most probably located within its orbital path. Subshells are designated by the letter s, p , d , and f . The s subshell is spherical in shape and has one orbital. Principal shell 1n has only a single s orbital, which can hold two electrons. Principal shell 2n has one s and one p subshell, and can hold a total of eight electrons. The p subshell has three dumbbell-shaped orbitals, as illustrated in [link] . Subshells d and f have more complex shapes and contain five and seven orbitals, respectively. These are not shown in the illustration. Principal shell 3n has s , p , and d subshells and can hold 18 electrons. Principal shell 4n has s , p , d and f orbitals and can hold 32 electrons. Moving away from the nucleus, the number of electrons and orbitals found in the energy levels increases. Progressing from one atom to the next in the periodic table, the electron structure can be worked out by fitting an extra electron into the next available orbital.
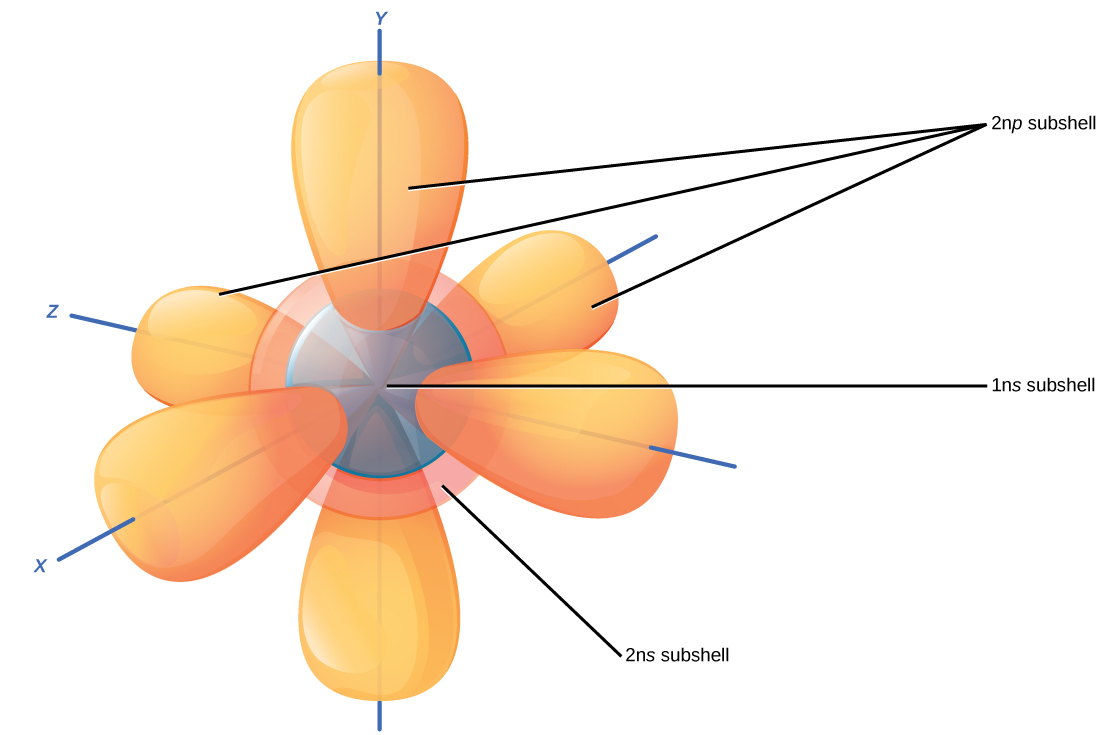
The closest orbital to the nucleus, called the 1s orbital, can hold up to two electrons. This orbital is equivalent to the innermost electron shell of the Bohr model of the atom. It is called the 1 s orbital because it is spherical around the nucleus. The 1 s orbital is the closest orbital to the nucleus, and it is always filled first, before any other orbital can be filled. Hydrogen has one electron; therefore, it has only one spot within the 1 s orbital occupied. This is designated as 1 s 1 , where the superscripted 1 refers to the one electron within the 1 s orbital. Helium has two electrons; therefore, it can completely fill the 1 s orbital with its two electrons. This is designated as 1 s 2 , referring to the two electrons of helium in the 1 s orbital. On the periodic table [link] , hydrogen and helium are the only two elements in the first row (period); this is because they only have electrons in their first shell, the 1 s orbital. Hydrogen and helium are the only two elements that have the 1 s and no other electron orbitals in the electrically neutral state.

Notification Switch
Would you like to follow the 'Biology' conversation and receive update notifications?