| << Chapter < Page | Chapter >> Page > |
For thousands of years, fear of the dead and legal sanctions limited the ability of anatomists and physicians to study the internal structures of the human body. An inability to control bleeding, infection, and pain made surgeries infrequent, and those that were performed—such as wound suturing, amputations, tooth and tumor removals, skull drilling, and cesarean births—did not greatly advance knowledge about internal anatomy. Theories about the function of the body and about disease were therefore largely based on external observations and imagination. During the fourteenth and fifteenth centuries, however, the detailed anatomical drawings of Italian artist and anatomist Leonardo da Vinci and Flemish anatomist Andreas Vesalius were published, and interest in human anatomy began to increase. Medical schools began to teach anatomy using human dissection; although some resorted to grave robbing to obtain corpses. Laws were eventually passed that enabled students to dissect the corpses of criminals and those who donated their bodies for research. Still, it was not until the late nineteenth century that medical researchers discovered non-surgical methods to look inside the living body.
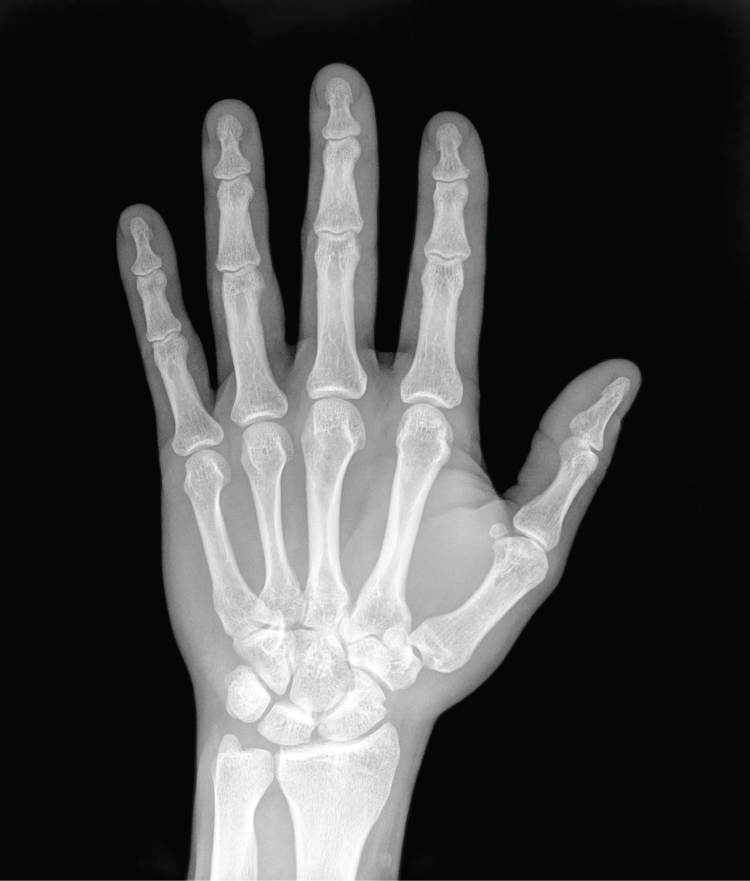
German physicist Wilhelm Röntgen (1845–1923) was experimenting with electrical current when he discovered that a mysterious and invisible “ray” would pass through his flesh but leave an outline of his bones on a screen coated with a metal compound. In 1895, Röntgen made the first durable record of the internal parts of a living human: an “X-ray” image (as it came to be called) of his wife’s hand. Scientists around the world quickly began their own experiments with X-rays, and by 1900, X-rays were widely used to detect a variety of injuries and diseases. In 1901, Röntgen was awarded the first Nobel Prize for physics for his work in this field.
The X-ray is a form of high energy electromagnetic radiation with a short wavelength capable of penetrating solids and ionizing gases. As they are used in medicine, X-rays are emitted from an X-ray machine and directed toward a specially treated metallic plate placed behind the patient’s body. The beam of radiation results in darkening of the X-ray plate. X-rays are slightly impeded by soft tissues, which show up as gray on the X-ray plate, whereas hard tissues, such as bone, largely block the rays, producing a light-toned “shadow.” Thus, X-rays are best used to visualize hard body structures such as teeth and bones ( [link] ). Like many forms of high energy radiation, however, X-rays are capable of damaging cells and initiating changes that can lead to cancer. This danger of excessive exposure to X-rays was not fully appreciated for many years after their widespread use.

Notification Switch
Would you like to follow the 'Anatomy & Physiology' conversation and receive update notifications?