| << Chapter < Page | Chapter >> Page > |
Once the information has been encoded, we have to somehow have to retain it. Our brains take the encoded information and place it in storage. Storage is the creation of a permanent record of information.
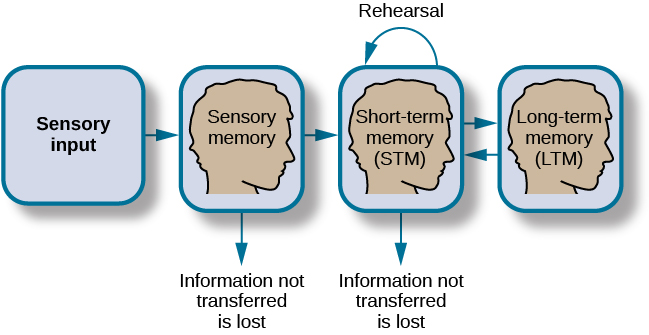
In order for a memory to go into storage (i.e., long-term memory), it has to pass through three distinct stages: Sensory Memory , Short-Term Memory , and finally Long-Term Memory . These stages were first proposed by Richard Atkinson and Richard Shiffrin (1968). Their model of human memory ( [link] ), called Atkinson-Shiffrin (A-S), is based on the belief that we process memories in the same way that a computer processes information.
But A-S is just one model of memory. Others, such as Baddeley and Hitch (1974), have proposed a model where short-term memory itself has different forms. In this model, storing memories in short-term memory is like opening different files on a computer and adding information. The type of short-term memory (or computer file) depends on the type of information received. There are memories in visual-spatial form, as well as memories of spoken or written material, and they are stored in three short-term systems: a visuospatial sketchpad, an episodic buffer, and a phonological loop. According to Baddeley and Hitch, a central executive part of memory supervises or controls the flow of information to and from the three short-term systems.
In the Atkinson-Shiffrin model, stimuli from the environment are processed first in sensory memory : storage of brief sensory events, such as sights, sounds, and tastes. It is very brief storage—up to a couple of seconds. We are constantly bombarded with sensory information. We cannot absorb all of it, or even most of it. And most of it has no impact on our lives. For example, what was your professor wearing the last class period? As long as the professor was dressed appropriately, it does not really matter what she was wearing. Sensory information about sights, sounds, smells, and even textures, which we do not view as valuable information, we discard. If we view something as valuable, the information will move into our short-term memory system.
One study of sensory memory researched the significance of valuable information on short-term memory storage. J. R. Stroop discovered a memory phenomenon in the 1930s: you will name a color more easily if it appears printed in that color, which is called the Stroop effect . In other words, the word “red” will be named more quickly, regardless of the color the word appears in, than any word that is colored red. Try an experiment: name the colors of the words you are given in [link] . Do not read the words, but say the color the word is printed in. For example, upon seeing the word “yellow” in green print, you should say “green,” not “yellow.” This experiment is fun, but it’s not as easy as it seems.


Notification Switch
Would you like to follow the 'Psychology' conversation and receive update notifications?