| << Chapter < Page | Chapter >> Page > |
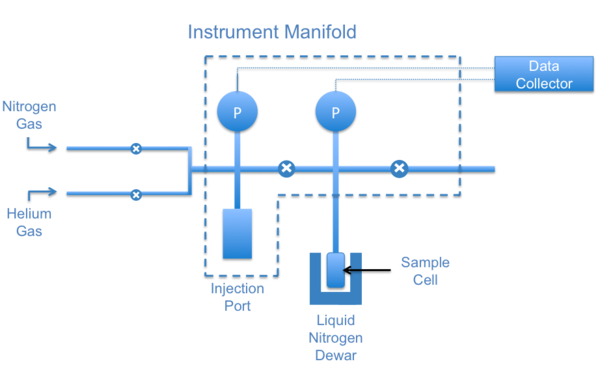
Samples are placed in glass cells to be degassed and analyzed by the BET machine. Glass rods are placed within the cell to minimize the dead space in the cell. Sample cells typically come in sizes of 6, 9 and 12 mm and come in different shapes. 6 mm cells are usually used for fine powders, 9 mm cells for larger particles and small pellets and 12 mm are used for large pieces that cannot be further reduced. The cells are placed into heating mantles and connected to the outgas port of the machine.
After the sample is degassed, the cell is moved to the analysis port ( [link] ). Dewars of liquid nitrogen are used to cool the sample and maintain it at a constant temperature. A low temperature must be maintained so that the interaction between the gas molecules and the surface of the sample will be strong enough for measurable amounts of adsorption to occur. The adsorbate, nitrogen gas in this case, is injected into the sample cell with a calibrated piston. The dead volume in the sample cell must be calibrated before and after each measurement. To do that, helium gas is used for a blank run, because helium does not adsorb onto the sample.
The BET technique has some disadvantages when compared to NMR, which can also be used to measure the surface area of nanoparticles. BET measurements can only be used to determine the surface area of dry powders. This technique requires a lot of time for the adsorption of gas molecules to occur. A lot of manual preparation is required.
The BET technique was used to determine the surface areas of metal-organic frameworks (MOFs), which are crystalline compounds of metal ions coordinated to organic molecules. Possible applications of MOFs, which are porous, include gas purification and catalysis. An isoreticular MOF (IRMOF) with the chemical formula Zn 4 O(pyrene-1,2-dicarboxylate) 3 ( [link] ) was used as an example to see if BET could accurately determine the surface area of microporous materials. The predicted surface area was calculated directly from the geometry of the crystals and agreed with the data obtained from the BET isotherms. Data was collected at a constant temperature of 77 K and a type II isotherm ( [link] ) was obtained.

The isotherm data obtained from partial pressure range of 0.05 to 0.3 is plugged into the BET equation, [link] , to obtain the BET plot ( [link] ).

Using [link] , the monolayer capacity is determined to be 391.2 cm 3 /g.
Now that X m is known, then [link] can be used to determine that the surface area is 1702.3 m 2 /g.

Notification Switch
Would you like to follow the 'Physical methods in chemistry and nano science' conversation and receive update notifications?