| << Chapter < Page | Chapter >> Page > |
Generally, the following reaction takes place in combustion analysis:
After burning 1.333 g of a hydrocarbon in a combustion analysis apparatus, 1.410 g of H 2 O and 4.305 g of CO 2 were produced. Separately, the molar mass of this hydrocarbon was found to be 204.35 g/mol. Calculate the empirical and molecular formulas of this hydrocarbon.
Step 1 : Using the molar masses of water and carbon dioxide, determine the moles of hydrogen and carbon that were produced.

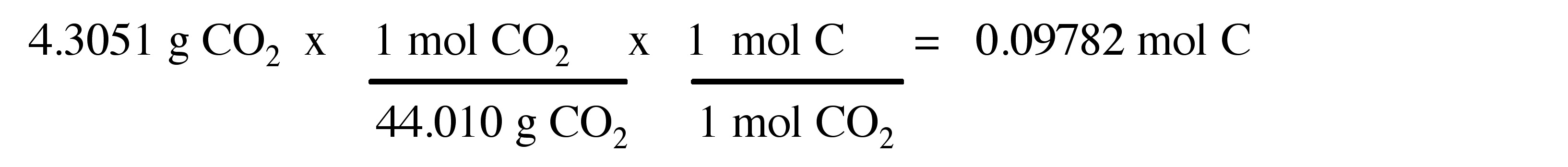
Step 2 : Divide the larger molar amount by the smaller molar amount. In some cases, the ratio is not made up of two integers. Convert the numerator of the ratio to an improper fraction and rewrite the ratio in whole numbers as shown.

Therefore, the empirical formula is C 5 H 8 .
Step 3 : To get the molecular formula, divide the experimental molar mass of the unknown hydrocarbon by the empirical formula weight.

Therefore, the molecular formula is (C 5 H 8 ) 3 or C 15 H 24 .
After burning 1.082 g of a hydrocarbon in a combustion analysis apparatus, 1.583 g of H 2 O and 3.315 g of CO 2 were produced. Separately, the molar mass of this hydrocarbon was found to be 258.52 g/mol. Calculate the empirical and molecular formulas of this hydrocarbon.
The empirical formula is C 3 H 7 , and the molecular formula is (C 3 H 7 ) 6 or C 18 H 42 .
Combustion analysis can also be utilized to determine the empiric and molecular formulas of compounds containing carbon, hydrogen, and oxygen. However, as the reaction is performed in an environment of excess oxygen, the amount of oxygen in the sample can be determined from the sample mass, rather than the combustion data ( [link] , [link] ).
A 2.0714 g sample containing carbon, hydrogen, and oxygen was burned in a combustion analysis apparatus; 1.928 g of H 2 O and 4.709 g of CO 2 were produced. Separately, the molar mass of the sample was found to be 116.16 g/mol. Determine the empirical formula, molecular formula, and identity of the sample.
Step 1 : Using the molar masses of water and carbon dioxide, determine the moles of hydrogen and carbon that were produced.


Step 2 : Using the molar amounts of carbon and hydrogen, calculate the masses of each in the original sample.


Step 3 : Subtract the masses of carbon and hydrogen from the sample mass. Now that the mass of oxygen is known, use this to calculate the molar amount of oxygen in the sample.

Step 4 : Divide each molar amount by the smallest molar amount in order to determine the ratio between the three elements.
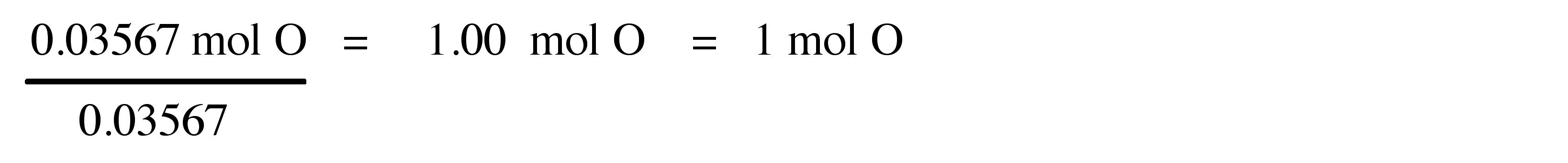


Therefore, the empirical formula is C 3 H 6 O.
Step 5 : To get the molecular formula, divide the experimental molar mass of the unknown hydrocarbon by the empirical formula weight.

Therefore, the molecular formula is (C 3 H 6 O) 2 or C 6 H 12 O 2 . Possible compound with this molecular formula are shown in ( [link] ).

A 4.846 g sample containing carbon, hydrogen, and oxygen was burned in a combustion analysis apparatus; 4.843 g of H 2 O and 11.83 g of CO 2 were produced. Separately, the molar mass of the sample was found to be 144.22 g/mol. Determine the empirical formula, molecular formula, and identity of the sample.
The empirical formula is C 4 H 8 O, and the molecular formula is (C 4 H 8 O) 2 or C 8 H 16 O 2 . Possible compounds with this molecular formula are shown in ( [link] ).
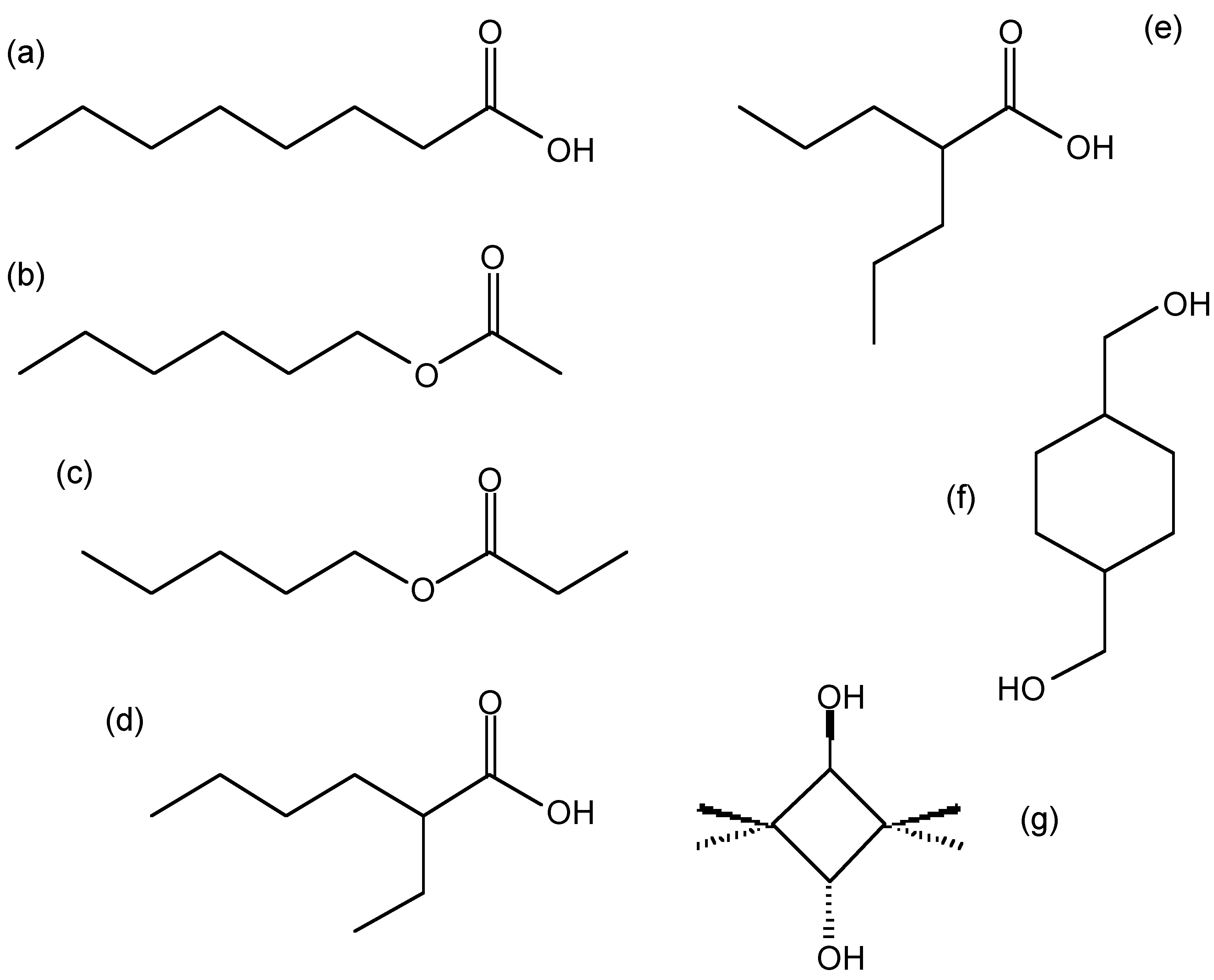
By using combustion analysis, the chemical formula of a binary compound containing oxygen can also be determined. This is particularly helpful in the case of combustion of a metal which can result in potential oxides of multiple oxidation states.
A sample of iron weighing 1.7480 g is combusted in the presence of excess oxygen. A metal oxide (Fe x O y ) is formed with a mass of 2.4982 g. Determine the chemical formula of the oxide product and the oxidation state of Fe.
Step 1 : Subtract the mass of Fe from the mass of the oxide to determine the mass of oxygen in the product.
Step 2 : Using the molar masses of Fe and O, calculate the molar amounts of each element.
Step 3 : Divide the larger molar amount by the smaller molar amount. In some cases, the ratio is not made up of two integers. Convert the numerator of the ratio to an improper fraction and rewrite the ratio in whole numbers as shown.
Therefore, the chemical formula of the oxide is Fe 2 O 3 , and Fe has a 3+ oxidation state.
A sample of copper weighing 7.295 g is combusted in the presence of excess oxygen. A metal oxide (Cu x O y ) is formed with a mass of 8.2131 g. Determine the chemical formula of the oxide product and the oxidation state of Cu.
The chemical formula is Cu 2 O, and Cu has a 1+ oxidation state.

Notification Switch
Would you like to follow the 'Physical methods in chemistry and nano science' conversation and receive update notifications?