| << Chapter < Page | Chapter >> Page > |
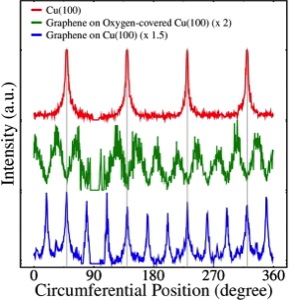
As previously mentioned, LEED–IV curves may give us exact information about the position of the atoms in a crystal. These curves are related to a variation of intensities of the diffracted electron (spots) with the energy of the electron beam. The process of determination of the structure by this technique works as ‘proof and error’ and consists of three main parts: the measurement of the intensity spectra, the calculations for various models of atomic positions and the search for the best-fit structure which is determined by an R-factor.
The first step consists of obtaining the experimental LEED pattern and all the electron beam intensities for every spot of the reciprocal lattice in the pattern. Theoretical LEED–IV curves are calculated for a large number of geometrical models and these are compared with the experimental curves. The agreement is quantified by means of a reliability factor or R–factor. The closest this value to zero is, the more perfect the agreement between experimental and theoretical curves. In this way, the level of precision of the crystalline structure will depend on the smallest R–factor that can be achieved.
Pure metals with pure surfaces allow R–factor values of around 0.1. When moving to more complex structures, these values increase. The main reason for this gradually worse agreement between theoretical and experimental LEED-IV curves lies in the approximations in conventional LEED theory, which treats the atoms as perfect spheres with constant scattering potential in between. This description results in inaccurate scattering potential for more open surfaces and organic molecules. In consequence, a precision of 1-2 pm can be achieved for atoms in metal surfaces, whereas the positions of atoms within organic molecules are typically determined within ±10-20 pm. The values of the R-factor are usually between 0.2 and 0.5, where 0.2 represents a good agreement, 0.35 a mediocre agreement and 0.5 a poor agreement.
[link] shows an example of a typical LEED–IV curve for Ir (100), which has a quasi-hexagonal unit cell. One can observe the parameters used to calculate the theoretical LEED–IV curve and the best-fitted curve obtained experimentally, which has an R–factor value of 0.144. The model used is also shown.

Notification Switch
Would you like to follow the 'Physical methods in chemistry and nano science' conversation and receive update notifications?