| << Chapter < Page | Chapter >> Page > |
where is the Bohr magneton and m is the angular momentum projection quantum number (or magnetic orbital quantum number ), which has the values
For example, in the electron state, the total energy of the electron is split into three distinct energy levels corresponding to
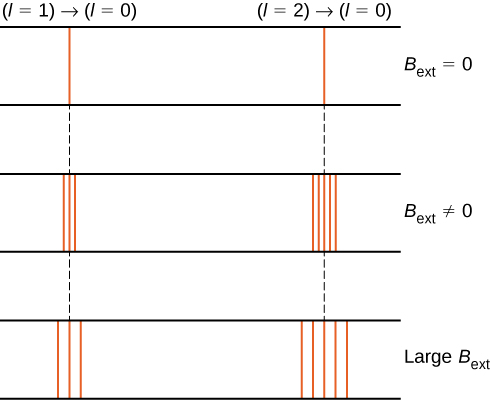
The splitting of energy levels by an external magnetic field is called the Zeeman effect . Ignoring the effects of electron spin, transitions from the state to a common lower energy state produce three closely spaced spectral lines ( [link] , left column). Likewise, transitions from the state produce five closely spaced spectral lines (right column). The separation of these lines is proportional to the strength of the external magnetic field. This effect has many applications. For example, the splitting of lines in the hydrogen spectrum of the Sun is used to determine the strength of the Sun’s magnetic field. Many such magnetic field measurements can be used to make a map of the magnetic activity at the Sun’s surface called a magnetogram ( [link] ).

Explain why spectral lines of the hydrogen atom are split by an external magnetic field. What determines the number and spacing of these lines?
A hydrogen atom is placed in a magnetic field. Which of the following quantities are affected? (a) total energy; (b) angular momentum; (c) z-component of angular momentum; (d) polar angle.
a, c, d; The total energy is changed (Zeeman splitting). The work done on the hydrogen atom rotates the atom, so the z -component of angular momentum and polar angle are affected. However, the angular momentum is not affected.
On what factors does the orbital magnetic dipole moment of an electron depend?
Find the magnitude of the orbital magnetic dipole moment of the electron in in the 3 p state. (Express your answer in terms of )
The 3 p state corresponds to , . Therefore,
A current of flows through a square-shaped wire with 2-cm side lengths. What is the magnetic moment of the wire?
Estimate the ratio of the electron magnetic moment to the muon magnetic moment for the same state of orbital angular momentum. ( Hint:
The ratio of their masses is 1/207, so the ratio of their magnetic moments is 207. The electron’s magnetic moment is more than 200 times larger than the muon.
Find the magnitude of the orbital magnetic dipole moment of the electron in in the 4 d state. (Express your answer in terms of )
For a 3 d electron in an external magnetic field of , find (a) the current associated with the orbital angular momentum, and (b) the maximum torque.
a. The 3
d state corresponds to
,
. So,
b. The maximum torque occurs when the magnetic moment and external magnetic field vectors are at right angles (
. In this case:
An electron in a hydrogen atom is in the , state. Find the smallest angle the magnetic moment makes with the z -axis. (Express your answer in terms of )
Find the minimum torque magnitude | that acts on the orbital magnetic dipole of a 3 p electron in an external magnetic field of .
A 3
p electron is in the state
and
. The minimum torque magnitude occurs when the magnetic moment and external magnetic field vectors are most parallel (antiparallel). This occurs when
.The torque magnitude is given by
Where
For
we have:
An electron in a hydrogen atom is in 3 p state. Find the smallest angle the magnetic moment makes with the z -axis. (Express your answer in terms of )
Show that .
( Hint : An infinitesimal amount of work is done to align the magnetic moment with the external field. This work rotates the magnetic moment vector through an angle (toward the positive z -direction), where is a positive angle change.)
An infinitesimal work
dW done by a magnetic torque
to rotate the magnetic moment through an angle
:
,
where
. Work done is interpreted as a drop in potential energy
U , so
The total energy change is determined by summing over infinitesimal changes in the potential energy:

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?