| << Chapter < Page | Chapter >> Page > |
| Element | Symbol | Mass Number | Mass (Atomic Mass Units) | Percent Abundance* | Half-life** |
|---|---|---|---|---|---|
| Hydrogen | H | 1 | 1.0078 | 99.99 | stable |
| 2 | 2.0141 | 0.01 | stable | ||
| 3 | 3.0160 | − | 12.32 y | ||
| Carbon | 12 | 12.0000 | 98.91 | stable | |
| 13 | 13.0034 | 1.1 | stable | ||
| 14 | 14.0032 | − | 5730 y | ||
| Nitrogen | 14 | 14.0031 | 99.6 | stable | |
| 15 | 15.0001 | 0.4 | stable | ||
| 16 | 16.0061 | − | 7.13 s | ||
| Oxygen | 16 | 15.9949 | 99.76 | stable | |
| 17 | 16.9991 | 0.04 | stable | ||
| 18 | 17.9992 | 0.20 | stable | ||
| 19 | 19.0035 | − | 26.46 s |
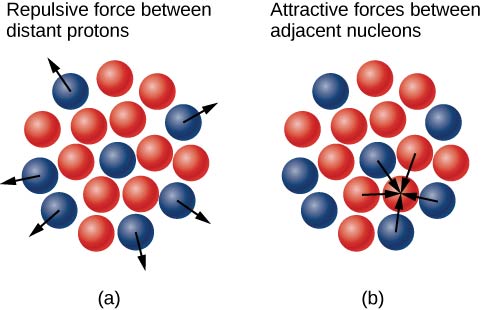
Why do neutrons outnumber protons in heavier nuclei ( [link] )? The answer to this question requires an understanding of forces inside the nucleus. Two types of forces exist: (1) the long-range electrostatic (Coulomb) force that makes the positively charged protons repel one another; and (2) the short-range strong nuclear force that makes all nucleons in the nucleus attract one another. You may also have heard of a “weak” nuclear force. This force is responsible for some nuclear decays, but as the name implies, it does not play a role in stabilizing the nucleus against the strong Coulomb repulsion it experiences. We discuss strong nuclear force in more detail in the next chapter when we cover particle physics. Nuclear stability occurs when the attractive forces between nucleons compensate for the repulsive, long-range electrostatic forces between all protons in the nucleus. For heavy nuclei excess neutrons are necessary to keep the electrostatic interactions from breaking the nucleus apart, as shown in [link] .
Because of the existence of stable isotopes, we must take special care when quoting the mass of an element. For example, Copper (Cu) has two stable isotopes:
Given these two “versions” of Cu, what is the mass of this element? The atomic mass of an element is defined as the weighted average of the masses of its isotopes. Thus, the atomic mass of Cu is The mass of an individual nucleus is often expressed in atomic mass unit s (u), where . (An atomic mass unit is defined as 1/12th the mass of a nucleus.) In atomic mass units, the mass of a helium nucleus ( A = 4 ) is approximately 4 u. A helium nucleus is also called an alpha ( α ) particle.
The simplest model of the nucleus is a densely packed sphere of nucleons. The volume V of the nucleus is therefore proportional to the number of nucleons A , expressed by
where r is the radius of a nucleus and k is a constant with units of volume. Solving for r , we have
where is a constant. For hydrogen ( ), corresponds to the radius of a single proton. Scattering experiments support this general relationship for a wide range of nuclei, and they imply that neutrons have approximately the same radius as protons. The experimentally measured value for is approximately 1.2 femtometer (recall that ).
Check Your Understanding Nucleus X is two times larger than nucleus Y. What is the ratio of their atomic masses?
eight
Define and make clear distinctions between the terms neutron, nucleon, nucleus, and nuclide.
The nucleus of an atom is made of one or more nucleons. A nucleon refers to either a proton or neutron. A nuclide is a stable nucleus.
What are isotopes? Why do isotopes of the same atom share the same chemical properties?
Find the atomic numbers, mass numbers, and neutron numbers for (a) (b) (c) (d) and (e) .
Use the rule
| Atomic Number ( Z ) | Neutron Number ( N ) | Mass Number ( A ) | |
|---|---|---|---|
| (a) | 29 | 29 | 58 |
| (b) | 11 | 13 | 24 |
| (c) | 84 | 126 | 210 |
| (d) | 20 | 25 | 45 |
| (e) | 82 | 124 | 206 |
Silver has two stable isotopes. The nucleus, has atomic mass 106.905095 g/mol with an abundance of ; whereas has atomic mass 108.904754 g/mol with an abundance of . Find the atomic mass of the element silver.
The mass ( M ) and the radius ( r ) of a nucleus can be expressed in terms of the mass number, A . (a) Show that the density of a nucleus is independent of A . (b) Calculate the density of a gold (Au) nucleus. Compare your answer to that for iron (Fe).
a.
;
b.
A particle has a mass equal to 10 u. If this mass is converted completely into energy, how much energy is released? Express your answer in mega-electron volts (MeV). (Recall that .)
Find the length of a side of a cube having a mass of 1.0 kg and the density of nuclear matter.
side length
The detail that you can observe using a probe is limited by its wavelength. Calculate the energy of a particle that has a wavelength of , small enough to detect details about one-tenth the size of a nucleon.

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?