| << Chapter < Page | Chapter >> Page > |
where the energies are given by [link] .
The quantum particle in a box model has practical applications in a relatively newly emerged field of optoelectronics, which deals with devices that convert electrical signals into optical signals. This model also deals with nanoscale physical phenomena, such as a nanoparticle trapped in a low electric potential bounded by high-potential barriers.
Using the quantum particle in a box model, describe how the possible energies of the particle are related to the size of the box.
Is it possible that when we measure the energy of a quantum particle in a box, the measurement may return a smaller value than the ground state energy? What is the highest value of the energy that we can measure for this particle?
No. For an infinite square well, the spacing between energy levels increases with the quantum number n . The smallest energy measured corresponds to the transition from n = 2 to 1, which is three times the ground state energy. The largest energy measured corresponds to a transition from to 1, which is infinity. (Note: Even particles with extremely large energies remain bound to an infinite square well—they can never “escape”)
For a quantum particle in a box, the first excited state has zero value at the midpoint position in the box, so that the probability density of finding a particle at this point is exactly zero. Explain what is wrong with the following reasoning: “If the probability of finding a quantum particle at the midpoint is zero, the particle is never at this point, right? How does it come then that the particle can cross this point on its way from the left side to the right side of the box?
Assume that an electron in an atom can be treated as if it were confined to a box of width . What is the ground state energy of the electron? Compare your result to the ground state kinetic energy of the hydrogen atom in the Bohr’s model of the hydrogen atom.
9.4 eV, 64%
Assume that a proton in a nucleus can be treated as if it were confined to a one-dimensional box of width 10.0 fm. (a) What are the energies of the proton when it is in the states corresponding to , , and ? (b) What are the energies of the photons emitted when the proton makes the transitions from the first and second excited states to the ground state?
An electron confined to a box has the ground state energy of 2.5 eV. What is the width of the box?
0.38 nm
What is the ground state energy (in eV) of a proton confined to a one-dimensional box the size of the uranium nucleus that has a radius of approximately 15.0 fm?
What is the ground state energy (in eV) of an -particle confined to a one-dimensional box the size of the uranium nucleus that has a radius of approximately 15.0 fm?
1.82 MeV
To excite an electron in a one-dimensional box from its first excited state to its third excited state requires 20.0 eV. What is the width of the box?
An electron confined to a box of width 0.15 nm by infinite potential energy barriers emits a photon when it makes a transition from the first excited state to the ground state. Find the wavelength of the emitted photon.
24.7 nm
If the energy of the first excited state of the electron in the box is 25.0 eV, what is the width of the box?
Suppose an electron confined to a box emits photons. The longest wavelength that is registered is 500.0 nm. What is the width of the box?
Hydrogen molecules are kept at 300.0 K in a cubical container with a side length of 20.0 cm. Assume that you can treat the molecules as though they were moving in a one-dimensional box. (a) Find the ground state energy of the hydrogen molecule in the container. (b) Assume that the molecule has a thermal energy given by and find the corresponding quantum number n of the quantum state that would correspond to this thermal energy.
An electron is confined to a box of width 0.25 nm. (a) Draw an energy-level diagram representing the first five states of the electron. (b) Calculate the wavelengths of the emitted photons when the electron makes transitions between the fourth and the second excited states, between the second excited state and the ground state, and between the third and the second excited states.
a.
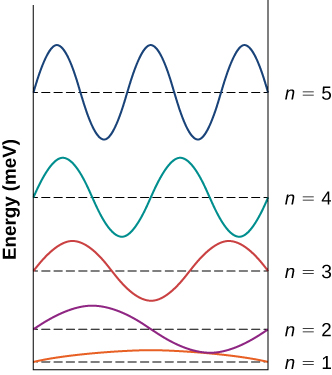 ;
;
b.
An electron in a box is in the ground state with energy 2.0 eV. (a) Find the width of the box. (b) How much energy is needed to excite the electron to its first excited state? (c) If the electron makes a transition from an excited state to the ground state with the simultaneous emission of 30.0-eV photon, find the quantum number of the excited state?

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?