| << Chapter < Page | Chapter >> Page > |
First, the periodic table is arranged into columns and rows. The table is read left to right and top to bottom in the order of increasing atomic number Z . Atoms that belong to the same column or chemical group share many of the same chemical properties. For example, the Li and Na atoms (in the first column) bond to other atoms in a similar way. The first row of the table corresponds to the 1 s ( ) shell of an atom.
Consider the hypothetical procedure of adding electrons, one by one, to an atom. For hydrogen (H) (upper left), the 1 s shell is filled with either a spin up or down electron ( ). This lone electron is easily shared with other atoms, so hydrogen is chemically active. For helium (He) (upper right), the 1 s shell is filled with both a spin up and a spin down ( ) electron. This “fills” the 1 s shell, so a helium atom tends not to share electrons with other atoms. The helium atom is said to be chemically inactive, inert, or noble; likewise, helium gas is said to be an inert gas or noble gas.
Build an atom by adding and subtracting protons, neutrons, and electrons. How does the element, charge, and mass change? Visit PhET Explorations: Build an Atom to explore the answers to these questions.
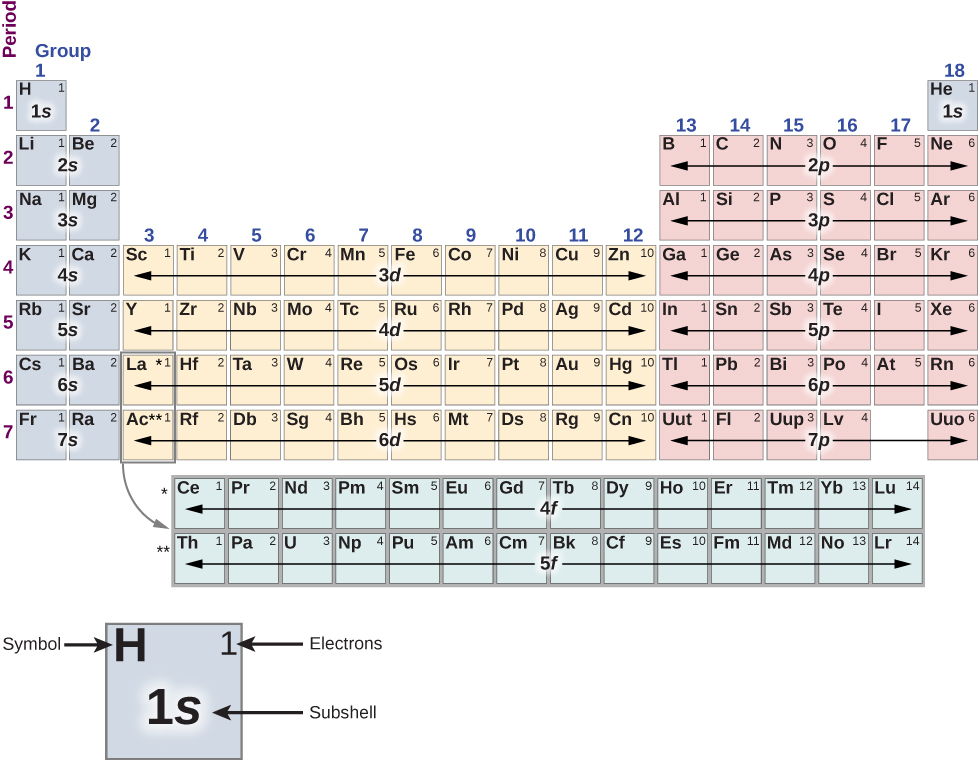
The second row corresponds to the 2 s and 2 p subshells. For lithium (Li) (upper left), the 1 s shell is filled with a spin-up and spin-down electron ( ) and the 2 s shell is filled with either a spin-up or -down electron ( ). Its electron configuration is therefore or [He]2 s , where [He] indicates a helium core. Like hydrogen, the lone electron in the outermost shell is easily shared with other atoms. For beryllium (Be), the 2 s shell is filled with a spin-up and -down electron ( ), and has the electron configuration [He] .
Next, we look at the right side of the table. For boron (B), the 1 s and 2 s shells are filled and the 2 p ( ) shell contains either a spin up or down electron ( ). From carbon (C) to neon (N), we the fill the 2 p shell. The maximum number of electrons in the 2 p shells is . For neon (Ne), the 1 s shell is filled with a spin-up and spin-down electron ( ), and the 2 p shell is filled with six electrons ( . This “fills” the 1 s , 2 s , and 2 p subshells, so like helium, the neon atom tends not to share electrons with other atoms.
The process of electron filling repeats in the third row. However, beginning in the fourth row, the pattern is broken. The actual order of order of electron filling is given by
1 s , 2 s , 2 p , 3 s , 3 p , 4 s , 3 d , 4 p , 5 s , 4 d , 5 p , 6 s , 4 f , 5 d , 6 p , 7 s ,...
Notice that the 3 d , 4 d , 4 f , and 5 d subshells (in bold) are filled out of order; this occurs because of interactions between electrons in the atom, which so far we have neglected. The transition metal s are elements in the gap between the first two columns and the last six columns that contain electrons that fill the d ( ) subshell. As expected, these atoms are arranged in columns. The structure of the periodic table can be understood in terms of the quantization of the total energy ( n ), orbital angular momentum ( l ), and spin ( s ). The first two columns correspond to the s ) subshell, the next six columns correspond to the p ( ) subshell, and the gap between these columns corresponds to the d ( ) subshell.

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?