| << Chapter < Page | Chapter >> Page > |
The probability of finding the electron in the region r to (“at approximately r ”) is
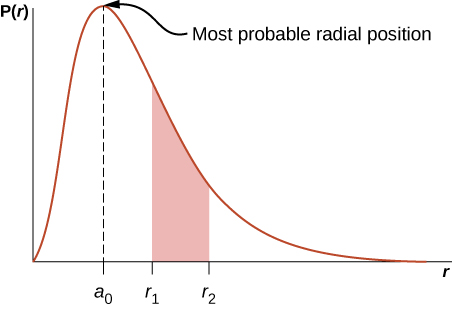
Here P ( r ) is called the radial probability density function (a probability per unit length). For an electron in the ground state of hydrogen, the probability of finding an electron in the region r to is
where angstroms. The radial probability density function P ( r ) is plotted in [link] . The area under the curve between any two radial positions, say and , gives the probability of finding the electron in that radial range. To find the most probable radial position, we set the first derivative of this function to zero ( ) and solve for r . The most probable radial position is not equal to the average or expectation value of the radial position because is not symmetrical about its peak value.
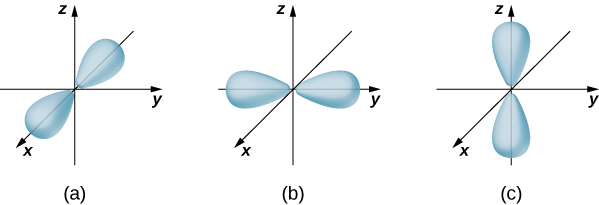
If the electron has orbital angular momentum ( ), then the wave functions representing the electron depend on the angles and that is, ( r , , ). Atomic orbitals for three states with and are shown in [link] . An atomic orbital is a region in space that encloses a certain percentage (usually 90%) of the electron probability. (Sometimes atomic orbitals are referred to as “clouds” of probability.) Notice that these distributions are pronounced in certain directions. This directionality is important to chemists when they analyze how atoms are bound together to form molecules.
A slightly different representation of the wave function is given in [link] . In this case, light and dark regions indicate locations of relatively high and low probability, respectively. In contrast to the Bohr model of the hydrogen atom, the electron does not move around the proton nucleus in a well-defined path. Indeed, the uncertainty principle makes it impossible to know how the electron gets from one place to another.

Identify the physical significance of each of the quantum numbers of the hydrogen atom.
n (principal quantum number)
total energy
(orbital angular quantum number)
total absolute magnitude of the orbital angular momentum
m (orbital angular projection quantum number)
z -component of the orbital angular momentum

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?