| << Chapter < Page | Chapter >> Page > |
Computing these derivatives leads to
According to de Broglie, so this expression implies that the total energy is equal to the kinetic energy, consistent with our assumption that the “particle moves freely.” Combining the results of [link] and [link] gives
Strange! A particle bound to a one-dimensional box can only have certain discrete (quantized) values of energy. Further, the particle cannot have a zero kinetic energy—it is impossible for a particle bound to a box to be “at rest.”
To evaluate the allowed wave functions that correspond to these energies, we must find the normalization constant . We impose the normalization condition [link] on the wave function
Hence, the wave functions that correspond to the energy values given in [link] are
For the lowest energy state or ground state energy , we have
All other energy states can be expressed as
The index n is called the energy quantum number or principal quantum number . The state for is the first excited state, the state for is the second excited state, and so on. The first three quantum states (for of a particle in a box are shown in [link] .
The wave functions in [link] are sometimes referred to as the “states of definite energy.” Particles in these states are said to occupy energy levels , which are represented by the horizontal lines in [link] . Energy levels are analogous to rungs of a ladder that the particle can “climb” as it gains or loses energy.
The wave functions in [link] are also called stationary state s and standing wave state s . These functions are “stationary,” because their probability density functions, , do not vary in time, and “standing waves” because their real and imaginary parts oscillate up and down like a standing wave—like a rope waving between two children on a playground. Stationary states are states of definite energy [ [link] ], but linear combinations of these states, such as (also solutions to Schrӧdinger’s equation) are states of mixed energy.
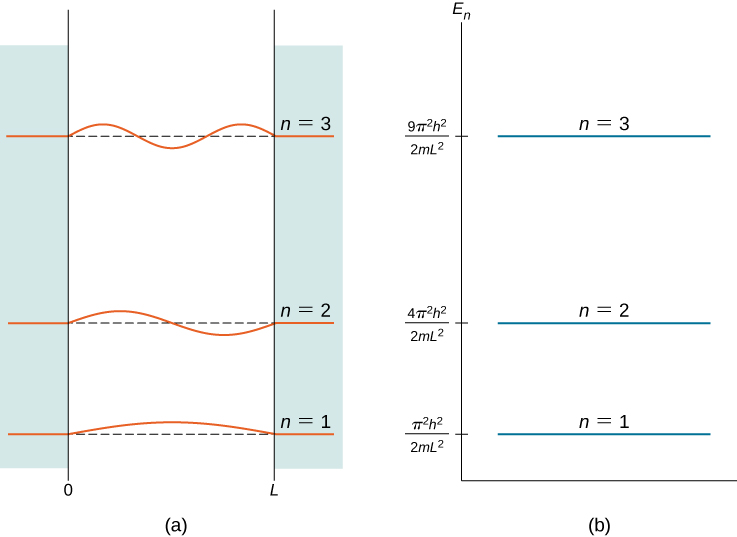
Energy quantization is a consequence of the boundary conditions. If the particle is not confined to a box but wanders freely, the allowed energies are continuous. However, in this case, only certain energies …) are allowed. The energy difference between adjacent energy levels is given by
Conservation of energy demands that if the energy of the system changes, the energy difference is carried in some other form of energy. For the special case of a charged particle confined to a small volume (for example, in an atom), energy changes are often carried away by photons. The frequencies of the emitted photons give us information about the energy differences (spacings) of the system and the volume of containment—the size of the “box” [see [link] ].

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?