| << Chapter < Page | Chapter >> Page > |
Total energy E of a particle is
where m is mass, c is the speed of light, and u is the velocity of the mass relative to an observer.
Rest energy of an object is
This is the correct form of Einstein’s most famous equation, which for the first time showed that energy is related to the mass of an object at rest. For example, if energy is stored in the object, its rest mass increases. This also implies that mass can be destroyed to release energy. The implications of these first two equations regarding relativistic energy are so broad that they were not completely recognized for some years after Einstein published them in 1905, nor was the experimental proof that they are correct widely recognized at first. Einstein, it should be noted, did understand and describe the meanings and implications of his theory.
Today, the practical applications of the conversion of mass into another form of energy , such as in nuclear weapons and nuclear power plants, are well known. But examples also existed when Einstein first proposed the correct form of relativistic energy, and he did describe some of them. Nuclear radiation had been discovered in the previous decade, and it had been a mystery as to where its energy originated. The explanation was that, in some nuclear processes, a small amount of mass is destroyed and energy is released and carried by nuclear radiation. But the amount of mass destroyed is so small that it is difficult to detect that any is missing. Although Einstein proposed this as the source of energy in the radioactive salts then being studied, it was many years before there was broad recognition that mass could be and, in fact, commonly is, converted to energy ( [link] ).
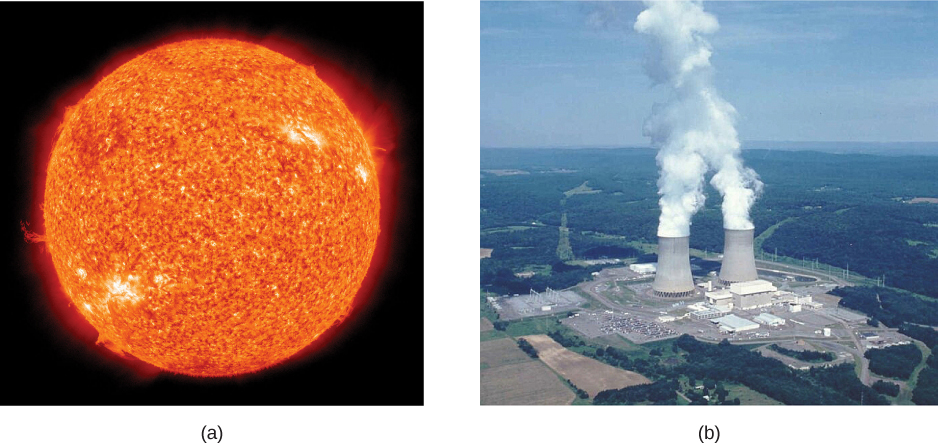
Because of the relationship of rest energy to mass, we now consider mass to be a form of energy rather than something separate. There had not been even a hint of this prior to Einstein’s work. Energy-mass equivalence is now known to be the source of the sun’s energy, the energy of nuclear decay, and even one of the sources of energy keeping Earth’s interior hot.

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?