-
Home
- University physics volume 2
- Unit 1. thermodynamics
- The first law of thermodynamics
- Thermodynamic processes
Many other processes also occur that do not fit into any of these four categories.
Summary
- The thermal behavior of a system is described in terms of thermodynamic variables. For an ideal gas, these variables are pressure, volume, temperature, and number of molecules or moles of the gas.
- For systems in thermodynamic equilibrium, the thermodynamic variables are related by an equation of state.
- A heat reservoir is so large that when it exchanges heat with other systems, its temperature does not change.
- A quasi-static process takes place so slowly that the system involved is always in thermodynamic equilibrium.
- A reversible process is one that can be made to retrace its path and both the temperature and pressure are uniform throughout the system.
- There are several types of thermodynamic processes, including (a) isothermal, where the system’s temperature is constant; (b) adiabatic, where no heat is exchanged by the system; (c) isobaric, where the system’s pressure is constant; and (d) isochoric, where the system’s volume is constant.
- As a consequence of the first law of thermodymanics, here is a summary of the thermodymaic processes: (a) isothermal:
(b) adiabatic:
(c) isobaric:
and (d) isochoric:
Conceptual questions
When a gas expands isothermally, it does work. What is the source of energy needed to do this work?
The system must be in contact with a heat source that allows heat to flow into the system.
Got questions? Get instant answers now!
It is unlikely that a process can be isothermal unless it is a very slow process. Explain why. Is the same true for isobaric and isochoric processes? Explain your answer.
Isothermal processes must be slow to make sure that as heat is transferred, the temperature does not change. Even for isobaric and isochoric processes, the system must be in thermal equilibrium with slow changes of thermodynamic variables.
Got questions? Get instant answers now!
Problems
Two moles of a monatomic ideal gas at (5 MPa, 5 L) is expanded isothermally until the volume is doubled (step 1). Then it is cooled isochorically until the pressure is 1 MPa (step 2). The temperature drops in this process. The gas is now compressed isothermally until its volume is back to 5 L, but its pressure is now 2 MPa (step 3). Finally, the gas is heated isochorically to return to the initial state (step 4). (a) Draw the four processes in the pV plane. (b) Find the total work done by the gas.
Got questions? Get instant answers now!
Consider a transformation from point
A to
B in a two-step process. First, the pressure is lowered from 3 MPa at point
A to a pressure of 1 MPa, while keeping the volume at 2 L by cooling the system. The state reached is labeled
C . Then the system is heated at a constant pressure to reach a volume of 6 L in the state
B . (a) Find the amount of work done on the
ACB path. (b) Find the amount of heat exchanged by the system when it goes from
A to
B on the
ACB path. (c) Compare the change in the internal energy when the
AB process occurs adiabatically with the
AB change through the two-step process on the
ACB path.
a. 1660 J; b. −2730 J; c. It does not depend on the process.
Got questions? Get instant answers now!
Consider a cylinder with a movable piston containing n moles of an ideal gas. The entire apparatus is immersed in a constant temperature bath of temperature T kelvin. The piston is then pushed slowly so that the pressure of the gas changes quasi-statically from
to
at constant temperature T. Find the work done by the gas in terms of n, R, T,
and
Got questions? Get instant answers now!
An ideal gas expands isothermally along AB and does 700 J of work (see below). (a) How much heat does the gas exchange along AB? (b) The gas then expands adiabatically along BC and does 400 J of work. When the gas returns to A along CA, it exhausts 100 J of heat to its surroundings. How much work is done on the gas along this path?
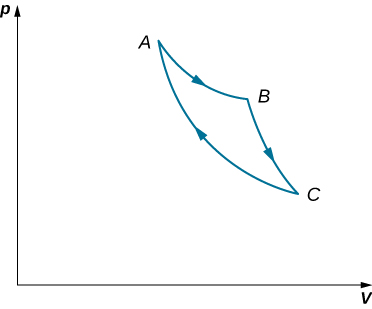
Got questions? Get instant answers now!
Consider the processes shown below. In the processes AB and BC, 3600 J and 2400 J of heat are added to the system, respectively. (a) Find the work done in each of the processes AB, BC, AD, and DC. (b) Find the internal energy change in processes AB and BC. (c) Find the internal energy difference between states C and A. (d) Find the total heat added in the ADC process. (e) From the information give, can you find the heat added in process AD? Why or why not?
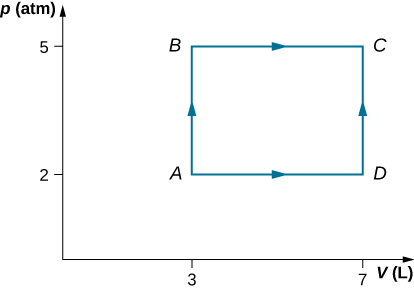
Got questions? Get instant answers now!
Two moles of helium gas are placed in a cylindrical container with a piston. The gas is at room temperature
and under a pressure of
When the pressure from the outside is decreased while keeping the temperature the same as the room temperature, the volume of the gas doubles. (a) Find the work the external agent does on the gas in the process. (b) Find the heat exchanged by the gas and indicate whether the gas takes in or gives up heat. Assume ideal gas behavior.
a. −3 400 J; b. 3400 J enters the gas
Got questions? Get instant answers now!
An amount of n moles of a monatomic ideal gas in a conducting container with a movable piston is placed in a large thermal heat bath at temperature
and the gas is allowed to come to equilibrium. After the equilibrium is reached, the pressure on the piston is lowered so that the gas expands at constant temperature. The process is continued quasi-statically until the final pressure is 4/3 of the initial pressure
(a) Find the change in the internal energy of the gas. (b) Find the work done by the gas. (c) Find the heat exchanged by the gas, and indicate, whether the gas takes in or gives up heat.
Got questions? Get instant answers now!
Questions & Answers
what does preconceived mean
How can I develope my cognitive domain
why is communication effective
Communication is effective because it allows individuals to share ideas, thoughts, and information with others.
effective communication can lead to improved outcomes in various settings, including personal relationships, business environments, and educational settings. By communicating effectively, individuals can negotiate effectively, solve problems collaboratively, and work towards common goals.
it starts up serve and return practice/assessments.it helps find voice talking therapy also assessments through relaxed conversation.
miss
Every time someone flushes a toilet in the apartment building, the person begins to jumb back automatically after hearing the flush, before the water temperature changes. Identify the types of learning, if it is classical conditioning identify the NS, UCS, CS and CR. If it is operant conditioning, identify the type of consequence positive reinforcement, negative reinforcement or punishment
please i need answer
Wekolamo
because it helps many people around the world to understand how to interact with other people and understand them well, for example at work (job).
Agreed 👍 There are many parts of our brains and behaviors, we really need to get to know. Blessings for everyone and happy Sunday!
ARC
A child is a member of community not society elucidate ?
Isn't practices worldwide, be it psychology, be it science. isn't much just a false belief of control over something the mind cannot truly comprehend?
compare and contrast skinner's perspective on personality development on freud
Skinner skipped the whole unconscious phenomenon and rather emphasized on classical conditioning
war
explain how nature and nurture affect the development and later the productivity of an individual.
nature is an hereditary factor while nurture is an environmental factor which constitute an individual personality. so if an individual's parent has a deviant behavior and was also brought up in an deviant environment, observation of the behavior and the inborn trait we make the individual deviant.
Samuel
I am taking this course because I am hoping that I could somehow learn more about my chosen field of interest and due to the fact that being a PsyD really ignites my passion as an individual the more I hope to learn about developing and literally explore the complexity of my critical thinking skills
and having a good philosophy of the world is like a sandwich and a peanut butter 👍
Jonathan
generally amnesi how long yrs memory loss
interpersonal relationships
What would be the best educational aid(s) for gifted kids/savants?
treat them normal, if they want help then give them.
that will make everyone happy
Saurabh
What are the treatment for autism?
hello. autism is a umbrella term. autistic kids have different disorder overlapping. for example. a kid may show symptoms of ADHD and also learning disabilities.
before treatment please make sure the kid doesn't have physical disabilities like hearing..vision..speech problem. sometimes these
Jharna
continue..
sometimes due to these physical problems..the diagnosis may be misdiagnosed.
treatment for autism.
well it depends on the severity.
since autistic kids have problems in communicating and adopting to the environment.. it's best to expose the child in situations where the child
Jharna
child interact with other kids under doc supervision.
play therapy.
speech therapy.
Engaging in different activities that activate most parts of the brain.. like drawing..painting. matching color board game.
string and beads game.
the more you interact with the child the more effective
Jharna
results you'll get..
please consult a therapist to know what suits best on your child.
and last as a parent. I know sometimes it's overwhelming to guide a special kid.
but trust the process and be strong and patient as a parent.
Jharna
Got questions? Join the online conversation and get instant answers!
Source:
OpenStax, University physics volume 2. OpenStax CNX. Oct 06, 2016 Download for free at http://cnx.org/content/col12074/1.3
Google Play and the Google Play logo are trademarks of Google Inc.


