| << Chapter < Page | Chapter >> Page > |
In this chapter, we study the electrical current through a material, where the electrical current is the rate of flow of charge. We also examine a characteristic of materials known as the resistance. Resistance is a measure of how much a material impedes the flow of charge, and it will be shown that the resistance depends on temperature. In general, a good conductor, such as copper, gold, or silver, has very low resistance. Some materials, called superconductors, have zero resistance at very low temperatures.
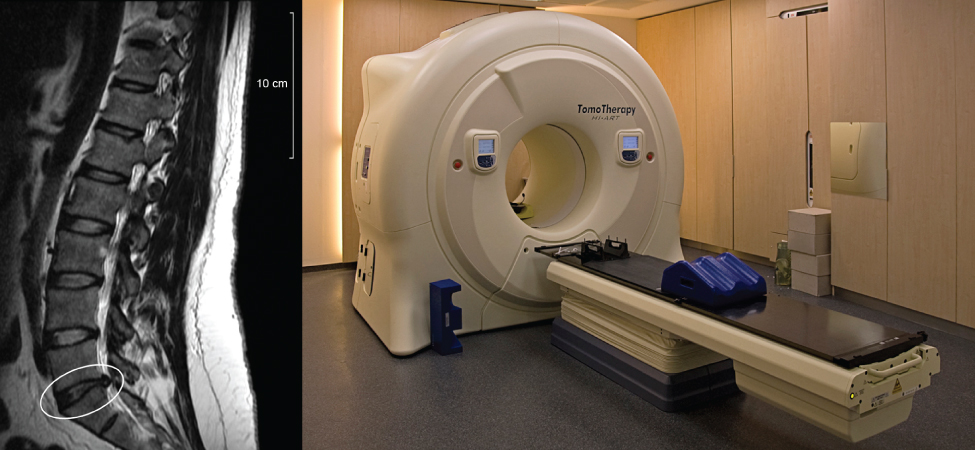
High currents are required for the operation of electromagnets. Superconductors can be used to make electromagnets that are 10 times stronger than the strongest conventional electromagnets. These superconducting magnets are used in the construction of magnetic resonance imaging (MRI) devices that can be used to make high-resolution images of the human body. The chapter-opening picture shows an MRI image of the vertebrae of a human subject and the MRI device itself. Superconducting magnets have many other uses. For example, superconducting magnets are used in the Large Hadron Collider (LHC) to curve the path of protons in the ring.

Notification Switch
Would you like to follow the 'University physics volume 2' conversation and receive update notifications?