| << Chapter < Page | Chapter >> Page > |
Check Your Understanding How many electrons would go through a 24.0-W lamp?
–2.00 C,
The energy per electron is very small in macroscopic situations like that in the previous example—a tiny fraction of a joule. But on a submicroscopic scale, such energy per particle (electron, proton, or ion) can be of great importance. For example, even a tiny fraction of a joule can be great enough for these particles to destroy organic molecules and harm living tissue. The particle may do its damage by direct collision, or it may create harmful X-rays, which can also inflict damage. It is useful to have an energy unit related to submicroscopic effects.
[link] shows a situation related to the definition of such an energy unit. An electron is accelerated between two charged metal plates, as it might be in an old-model television tube or oscilloscope. The electron gains kinetic energy that is later converted into another form—light in the television tube, for example. (Note that in terms of energy, “downhill” for the electron is “uphill” for a positive charge.) Since energy is related to voltage by , we can think of the joule as a coulomb-volt.
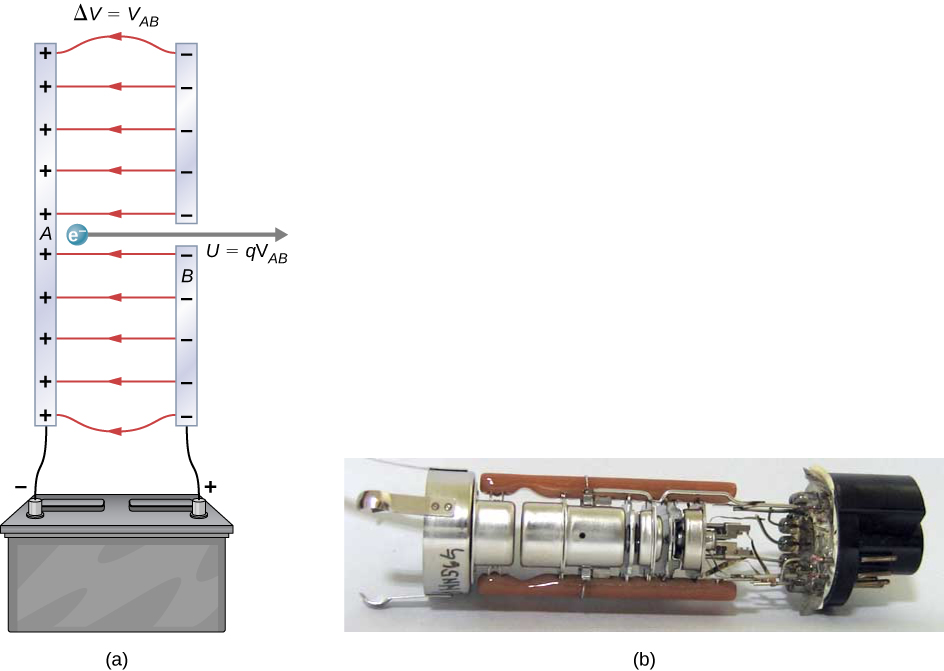
On the submicroscopic scale, it is more convenient to define an energy unit called the electron-volt (eV), which is the energy given to a fundamental charge accelerated through a potential difference of 1 V. In equation form,
An electron accelerated through a potential difference of 1 V is given an energy of 1 eV. It follows that an electron accelerated through 50 V gains 50 eV. A potential difference of 100,000 V (100 kV) gives an electron an energy of 100,000 eV (100 keV), and so on. Similarly, an ion with a double positive charge accelerated through 100 V gains 200 eV of energy. These simple relationships between accelerating voltage and particle charges make the electron-volt a simple and convenient energy unit in such circumstances.
The electron-volt is commonly employed in submicroscopic processes—chemical valence energies and molecular and nuclear binding energies are among the quantities often expressed in electron-volts. For example, about 5 eV of energy is required to break up certain organic molecules. If a proton is accelerated from rest through a potential difference of 30 kV, it acquires an energy of 30 keV (30,000 eV) and can break up as many as 6000 of these molecules Nuclear decay energies are on the order of 1 MeV (1,000,000 eV) per event and can thus produce significant biological damage.

Notification Switch
Would you like to follow the 'University physics volume 2' conversation and receive update notifications?