| << Chapter < Page | Chapter >> Page > |
In an isochoric process, heat is added to 10 mol of monoatomic ideal gas whose temperature increases from 273 to 373 K. What is the entropy change of the gas?
Two hundred grams of water at is brought into contact with a heat reservoir at . After thermal equilibrium is reached, what is the temperature of the water? Of the reservoir? How much heat has been transferred in the process? What is the entropy change of the water? Of the reservoir? What is the entropy change of the universe?
, , , 215 J/K, –190 J/K, 25 J/K
Suppose that the temperature of the water in the previous problem is raised by first bringing it to thermal equilibrium with a reservoir at a temperature of and then with a reservoir at . Calculate the entropy changes of (a) each reservoir, (b) of the water, and (c) of the universe.
Two hundred grams of water at is brought into contact into thermal equilibrium successively with reservoirs at , , , and . (a) What is the entropy change of the water? (b) Of the reservoir? (c) What is the entropy change of the universe?
, ,
(a) Ten grams of starts as ice at . The ice absorbs heat from the air (just above ) until all of it melts. Calculate the entropy change of the , of the air, and of the universe. (b) Suppose that the air in part (a) is at rather than and that the ice absorbs heat until it becomes water at . Calculate the entropy change of the , of the air, and of the universe. (c) Is either of these processes reversible?
The Carnot cycle is represented by the temperature-entropy diagram shown below. (a) How much heat is absorbed per cycle at the high-temperature reservoir? (b) How much heat is exhausted per cycle at the low-temperature reservoir? (c) How much work is done per cycle by the engine? (d) What is the efficiency of the engine?
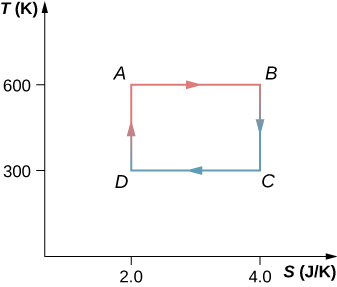
a. 1200 J; b. 600 J; c. 600 J; d. 0.50
A Carnot engine operating between heat reservoirs at 500 and 300 K absorbs 1500 J per cycle at the high-temperature reservoir. (a) Represent the engine’s cycle on a temperature-entropy diagram. (b) How much work per cycle is done by the engine?
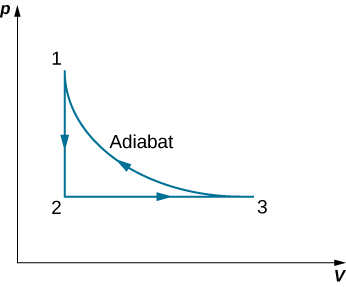
A monoatomic ideal gas ( n moles) goes through a cyclic process shown below. Find the change in entropy of the gas in each step and the total entropy change over the entire cycle.
A Carnot engine has an efficiency of 0.60. When the temperature of its cold reservoir changes, the efficiency drops to 0.55. If initially , determine (a) the constant value of and (b) the final value of .
A Carnot engine performs 100 J of work while rejecting 200 J of heat each cycle. After the temperature of the hot reservoir only is adjusted, it is found that the engine now does 130 J of work while discarding the same quantity of heat. (a) What are the initial and final efficiencies of the engine? (b) What is the fractional change in the temperature of the hot reservoir?
a. 0.33, 0.39; b. 0.91
A Carnot refrigerator exhausts heat to the air, which is at a temperature of . How much power is used by the refrigerator if it freezes 1.5 g of water per second? Assume the water is at .

Notification Switch
Would you like to follow the 'University physics volume 2' conversation and receive update notifications?