| << Chapter < Page | Chapter >> Page > |
This work is equal to the area enclosed by the loop shown in the pV diagram of [link] . Because the initial and final states of the system are the same, the change of the internal energy of the gas in the cycle must be zero, that is, . The first law of thermodynamics then gives
and
To find the efficiency of this engine, we first divide
When the adiabatic constant from step 2 is divided by that of step 4, we find
Substituting this into the equation for we obtain
Finally, with [link] , we find that the efficiency of this ideal gas Carnot engine is given by
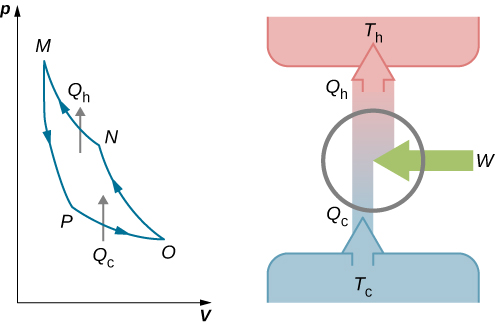
An engine does not necessarily have to follow a Carnot engine cycle. All engines, however, have the same net effect, namely the absorption of heat from a hot reservoir, the production of work, and the discarding of heat to a cold reservoir. This leads us to ask: Do all reversible cycles operating between the same two reservoirs have the same efficiency? The answer to this question comes from the second law of thermodynamics discussed earlier: All reversible engine cycles produce exactly the same efficiency . Also, as you might expect, all real engines operating between two reservoirs are less efficient than reversible engines operating between the same two reservoirs. This too is a consequence of the second law of thermodynamics shown earlier.
The cycle of an ideal gas Carnot refrigerator is represented by the pV diagram of [link] . It is a Carnot engine operating in reverse. The refrigerator extracts heat from a cold-temperature reservoir at when the ideal gas expands isothermally. The gas is then compressed adiabatically until its temperature reaches after which an isothermal compression of the gas results in heat being discarded to a high-temperature reservoir at Finally, the cycle is completed by an adiabatic expansion of the gas, causing its temperature to drop to
The work done on the ideal gas is equal to the area enclosed by the path of the pV diagram. From the first law, this work is given by
An analysis just like the analysis done for the Carnot engine gives
When combined with [link] , this yields
for the coefficient of performance of the ideal-gas Carnot refrigerator. Similarly, we can work out the coefficient of performance for a Carnot heat pump as
We have just found equations representing the efficiency of a Carnot engine and the coefficient of performance of a Carnot refrigerator or a Carnot heat pump, assuming an ideal gas for the working substance in both devices. However, these equations are more general than their derivations imply. We will soon show that they are both valid no matter what the working substance is.
Carnot summarized his study of the Carnot engine and Carnot cycle into what is now known as Carnot’s principle :
No engine working between two reservoirs at constant temperatures can have a greater efficiency than a reversible engine.

Notification Switch
Would you like to follow the 'University physics volume 2' conversation and receive update notifications?