| << Chapter < Page | Chapter >> Page > |
Four moles of a monatomic ideal gas in a cylinder at is expanded at constant pressure equal to 1 atm until its volume doubles. (a) What is the change in internal energy? (b) How much work was done by the gas in the process? (c) How much heat was transferred to the gas?
a. 15,000 J; b. 10,000 J; c. 25,000 J
Helium gas is cooled from to by expanding from 40 atm to 1 atm. If there is 1.4 mol of helium, (a) What is the final volume of helium? (b) What is the change in internal energy?
In an adiabatic process, oxygen gas in a container is compressed along a path that can be described by the following pressure in atm as a function of volume V, with : . The initial and final volumes during the process were 2 L and 1.5 L, respectively. Find the amount of work done on the gas.
78 J
A cylinder containing three moles of a monatomic ideal gas is heated at a constant pressure of 2 atm. The temperature of the gas changes from 300 K to 350 K as a result of the expansion. Find work done (a) on the gas; and (b) by the gas.
A cylinder containing three moles of nitrogen gas is heated at a constant pressure of 2 atm. The temperature of the gas changes from 300 K to 350 K as a result of the expansion. Find work done (a) on the gas, and (b) by the gas by using van der Waals equation of state instead of ideal gas law.
A cylinder containing three moles of nitrogen gas is heated at a constant pressure of 2 atm. a. − 1220 J; b. +1220 J
Two moles of a monatomic ideal gas such as oxygen is compressed adiabatically and reversibly from a state (3 atm, 5 L) to a state with a pressure of 4 atm. (a) Find the volume and temperature of the final state. (b) Find the temperature of the initial state. (c) Find work done by the gas in the process. (d) Find the change in internal energy in the process. Assume and for the diatomic ideal gas in the conditions given.
An insulated vessel contains 1.5 moles of argon at 2 atm. The gas initially occupies a volume of 5 L. As a result of the adiabatic expansion the pressure of the gas is reduced to 1 atm. (a) Find the volume and temperature of the final state. (b) Find the temperature of the gas in the initial state. (c) Find the work done by the gas in the process. (d) Find the change in the internal energy of the gas in the process.
a. 7.6 L, 61.6 K; b. 81.3 K; c. ; d. −367 J
One mole of an ideal monatomic gas occupies a volume of at a pressure of (a) What is the temperature of the gas? (b) The gas undergoes a quasi-static adiabatic compression until its volume is decreased to What is the new gas temperature? (c) How much work is done on the gas during the compression? (d) What is the change in the internal energy of the gas?
One mole of an ideal gas is initially in a chamber of volume and at a temperature of . (a) How much heat is absorbed by the gas when it slowly expands isothermally to twice its initial volume? (b) Suppose the gas is slowly transformed to the same final state by first decreasing the pressure at constant volume and then expanding it isobarically. What is the heat transferred for this case? (c) Calculate the heat transferred when the gas is transformed quasi-statically to the same final state by expanding it isobarically, then decreasing its pressure at constant volume.
a. 1700 J; b. 1200 J; c. 2400 J
A bullet of mass 10 g is traveling horizontally at 200 m/s when it strikes and embeds in a pendulum bob of mass 2.0 kg. (a) How much mechanical energy is dissipated in the collision? (b) Assuming that for the bob plus bullet is 3R, calculate the temperature increase of the system due to the collision. Take the molecular mass of the system to be 200 g/mol.
The insulated cylinder shown below is closed at both ends and contains an insulating piston that is free to move on frictionless bearings. The piston divides the chamber into two compartments containing gases A and B. Originally, each compartment has a volume of and contains a monatomic ideal gas at a temperature of and a pressure of 1.0 atm. (a) How many moles of gas are in each compartment? (b) Heat Q is slowly added to A so that it expands and B is compressed until the pressure of both gases is 3.0 atm. Use the fact that the compression of B is adiabatic to determine the final volume of both gases. (c) What are their final temperatures? (d) What is the value of Q?
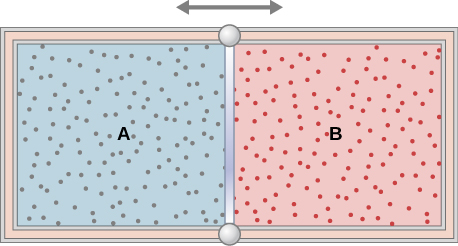
a. 2.2 mol; b. , ; c. ; d. 26,000 J
In a diesel engine, the fuel is ignited without a spark plug. Instead, air in a cylinder is compressed adiabatically to a temperature above the ignition temperature of the fuel; at the point of maximum compression, the fuel is injected into the cylinder. Suppose that air at is taken into the cylinder at a volume and then compressed adiabatically and quasi-statically to a temperature of and a volume If what is the ratio (Note: In an operating diesel engine, the compression is not quasi-static.)

Notification Switch
Would you like to follow the 'University physics volume 2' conversation and receive update notifications?