| << Chapter < Page | Chapter >> Page > |
In a sample of gas in a container, the randomness of the molecular motion causes the number of collisions of molecules with any part of the wall in a given time to fluctuate. However, because a huge number of molecules collide with the wall in a short time, the number of collisions on the scales of time and space we measure fluctuates by only a tiny, usually unobservable fraction from the average. We can compare this situation to that of a casino, where the outcomes of the bets are random and the casino’s takings fluctuate by the minute and the hour. However, over long times such as a year, the casino’s takings are very close to the averages expected from the odds. A tank of gas has enormously more molecules than a casino has bettors in a year, and the molecules make enormously more collisions in a second than a casino has bets.
A calculation of the average force exerted by molecules on the walls of the box leads us to the ideal gas law and to the connection between temperature and molecular kinetic energy. (In fact, we will take two averages: one over time to get the average force exerted by one molecule with a given velocity, and then another average over molecules with different velocities.) This approach was developed by Daniel Bernoulli (1700–1782), who is best known in physics for his work on fluid flow (hydrodynamics). Remarkably, Bernoulli did this work before Dalton established the view of matter as consisting of atoms.
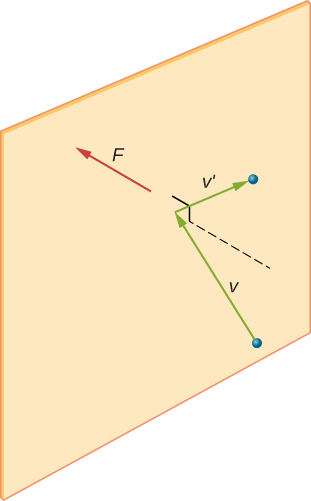
[link] shows a container full of gas and an expanded view of an elastic collision of a gas molecule with a wall of the container, broken down into components. We have assumed that a molecule is small compared with the separation of molecules in the gas, and that its interaction with other molecules can be ignored. Under these conditions, the ideal gas law is experimentally valid. Because we have also assumed the wall is rigid and the particles are points, the collision is elastic (by conservation of energy—there’s nowhere for a particle’s kinetic energy to go). Therefore, the molecule’s kinetic energy remains constant, and hence, its speed and the magnitude of its momentum remain constant as well. This assumption is not always valid, but the results in the rest of this module are also obtained in models that let the molecules exchange energy and momentum with the wall.
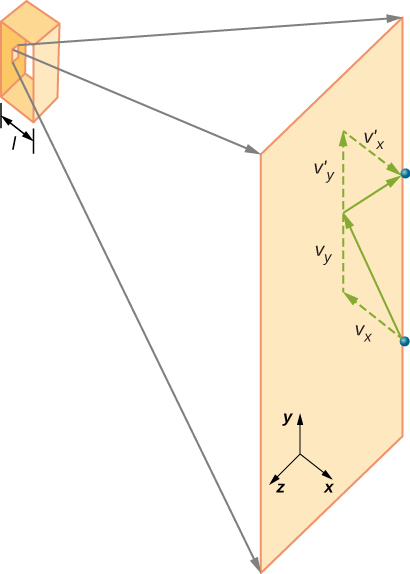
If the molecule’s velocity changes in the x -direction, its momentum changes from to Thus, its change in momentum is According to the impulse-momentum theorem given in the chapter on linear momentum and collisions, the force exerted on the i th molecule, where i labels the molecules from 1 to N , is given by

Notification Switch
Would you like to follow the 'University physics volume 2' conversation and receive update notifications?