| << Chapter < Page | Chapter >> Page > |
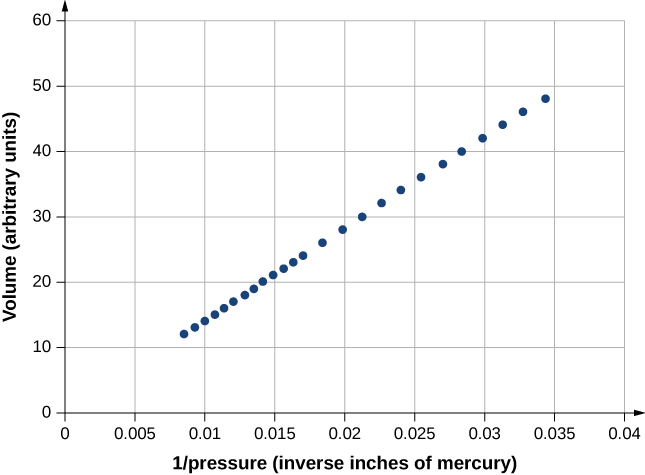
[link] shows data from the experiments of Robert Boyle (1627–1691), illustrating what is now called Boyle’s law : At constant temperature and number of molecules, the absolute pressure of a gas and its volume are inversely proportional. (Recall from Fluid Mechanics that the absolute pressure is the true pressure and the gauge pressure is the absolute pressure minus the ambient pressure, typically atmospheric pressure.) The graph in [link] displays this relationship as an inverse proportionality of volume to pressure.
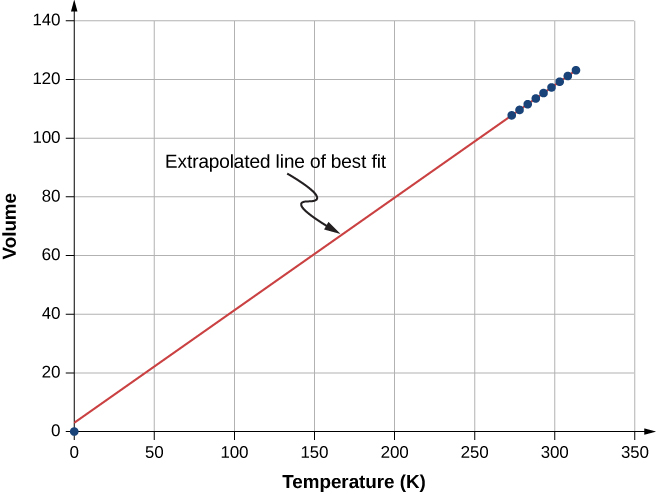
[link] shows experimental data illustrating what is called Charles’s law , after Jacques Charles (1746–1823). Charles’s law states that at constant pressure and number of molecules, the volume of a gas is proportional to its absolute temperature.
Similar is Amonton’s or Gay-Lussac’s law , which states that at constant volume and number of molecules, the pressure is proportional to the temperature. That law is the basis of the constant-volume gas thermometer, discussed in the previous chapter. (The histories of these laws and the appropriate credit for them are more complicated than can be discussed here.)
It is known experimentally that for gases at low density (such that their molecules occupy a negligible fraction of the total volume) and at temperatures well above the boiling point, these proportionalities hold to a good approximation. Not surprisingly, with the other quantities held constant, either pressure or volume is proportional to the number of molecules. More surprisingly, when the proportionalities are combined into a single equation, the constant of proportionality is independent of the composition of the gas. The resulting equation for all gases applies in the limit of low density and high temperature; it’s the same for oxygen as for helium or uranium hexafluoride. A gas at that limit is called an ideal gas ; it obeys the ideal gas law , which is also called the equation of state of an ideal gas.

Notification Switch
Would you like to follow the 'University physics volume 2' conversation and receive update notifications?