| << Chapter < Page | Chapter >> Page > |
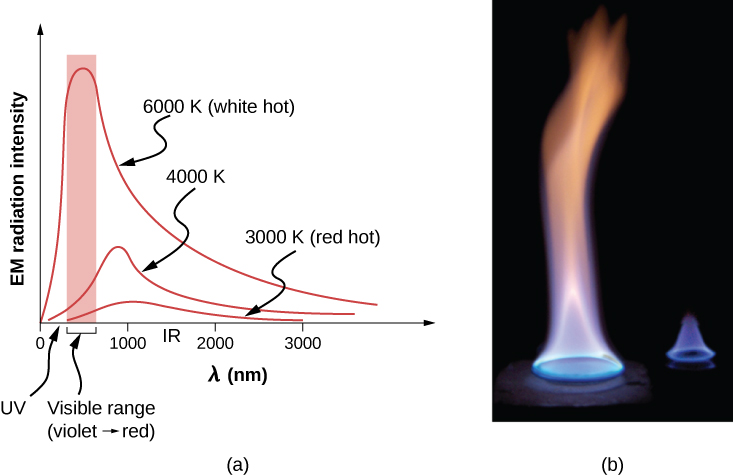
The rate of heat transfer by radiation also depends on the object’s color. Black is the most effective, and white is the least effective. On a clear summer day, black asphalt in a parking lot is hotter than adjacent gray sidewalk, because black absorbs better than gray ( [link] ). The reverse is also true—black radiates better than gray. Thus, on a clear summer night, the asphalt is colder than the gray sidewalk, because black radiates the energy more rapidly than gray. A perfectly black object would be an ideal radiator and an ideal absorber , as it would capture all the radiation that falls on it. In contrast, a perfectly white object or a perfect mirror would reflect all radiation, and a perfectly transparent object would transmit it all ( [link] ). Such objects would not emit any radiation. Mathematically, the color is represented by the emissivity e . A “blackbody” radiator would have an , whereas a perfect reflector or transmitter would have . For real examples, tungsten light bulb filaments have an e of about 0.5, and carbon black (a material used in printer toner) has an emissivity of about 0.95.

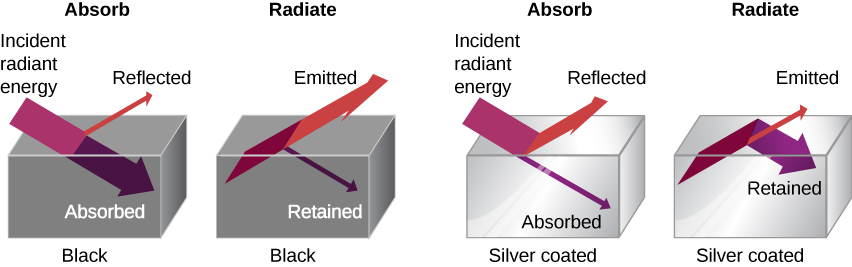
To see that, consider a silver object and a black object that can exchange heat by radiation and are in thermal equilibrium. We know from experience that they will stay in equilibrium (the result of a principle that will be discussed at length in Second Law of Thermodynamics ). For the black object’s temperature to stay constant, it must emit as much radiation as it absorbs, so it must be as good at radiating as absorbing. Similar considerations show that the silver object must radiate as little as it absorbs. Thus, one property, emissivity, controls both radiation and absorption.
Finally, the radiated heat is proportional to the object’s surface area, since every part of the surface radiates. If you knock apart the coals of a fire, the radiation increases noticeably due to an increase in radiating surface area.
The rate of heat transfer by emitted radiation is described by the Stefan-Boltzmann law of radiation :
where is the Stefan-Boltzmann constant, a combination of fundamental constants of nature; A is the surface area of the object; and T is its temperature in kelvins.

Notification Switch
Would you like to follow the 'University physics volume 2' conversation and receive update notifications?