| << Chapter < Page | Chapter >> Page > |
In "The Galvanic Cell" , we saw that a chemical reaction that involves a transfer of electrons, can be used to produce an electric current. In this section, we are going to see whether the 'reverse' process applies. In other words, is it possible to use an electric current to force a particular chemical reaction to occur, which would otherwise not take place? The answer is 'yes', and the type of cell that is used to do this, is called an electrolytic cell .
An electrolytic cell is a type of cell that uses electricity to drive a non-spontaneous reaction.
An electrolytic cell is activated by applying an electrical potential across the anode and cathode to force an internal chemical reaction between the ions that are in the electrolyte solution. This process is called electrolysis .
In chemistry and manufacturing, electrolysis is a method of separating bonded elements and compounds by passing an electric current through them.
A piece of filter paper is soaked in an ammonia-ammonium chloride solution and placed on a microscope slide. The filter paper is then connected to a supply of electric current using crocodile clips and connecting wire as shown in the diagram below. A line of copper chromate solution is placed in the centre of the filter paper. The colour of this solution is initially green-brown.

The current is then switched on and allowed to run for about 20 minutes. After this time, the central coloured band disappears and is replaced by two bands, one yellow and the other blue, which seem to have separated out from the first band of copper chromate.
Explanation:
Conclusion:
The movement of ions occurs because the electric current in the outer circuit sets up a potential difference between the two electrodes.
Similar principles apply in the electrolytic cell, where substances that are made of ions can be broken down into simpler substances through electrolysis.
There are a number of examples of electrolysis. The electrolysis of copper sulphate is just one.
Two copper electrodes are placed in a solution of blue copper sulphate and are connected to a source of electrical current as shown in the diagram below. The current is turned on and the reaction is left for a period of time.
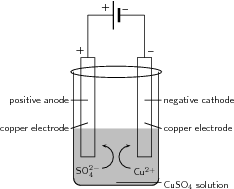
Observations:
Explanation:
Conclusion:
In this demonstration, an electric current was used to split CuSO into its component ions, and SO . This process is called electrolysis .
Water can also undergo electrolysis to form hydrogen gas and oxygen gas according to the following reaction:
This reaction is very important because hydrogen gas has the potential to be used as an energy source. The electrolytic cell for this reaction consists of two electrodes (normally platinum metal), submerged in an electrolyte and connected to a source of electric current.
The reduction half-reaction that takes place at the cathode is as follows:
The oxidation half-reaction that takes place at the anode is as follows:
It should be much clearer now that there are a number of differences between a galvanic and an electrolytic cell. Some of these differences have been summarised in [link] .
| Item | Galvanic cell | Electrolytic cell |
| Metals used for electrode | Two metals with different reaction potentials are used as electrodes | The same metal can be used for both the cathode and the anode |
| Charge of the anode | negative | positive |
| Charge of the cathode | positive | negative |
| The electrolyte solution/s | The electrolyte solutions are kept separate from one another, and are connected only by a salt bridge | The cathode and anode are in the same electrolyte |
| Energy changes | Chemical potential energy from chemical reactions is converted to electrical energy | An external supply of electrical energy causes a chemical reaction to occur |
| Applications | Run batteries, electroplating | Electrolysis e.g. of water, NaCl |

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 12 physical science' conversation and receive update notifications?