| << Chapter < Page | Chapter >> Page > |
Proteins are an incredibly important part of any cell, and they carry out a number of functions such as support, storage and transport within the body. The monomers of proteins are called amino acids . An amino acid is an organic molecule that contains a carboxyl and an amino group, as well as a carbon side chain. The carbon side chain varies from one amino acid to the next, and is sometimes simply represented by the letter 'R' in a molecule's structural formula. [link] shows some examples of different amino acids.
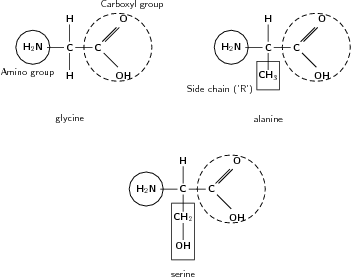
Although each of these amino acids has the same basic structure, their side chains ('R' groups) are different. In the amino acid glycine, the side chain consists only of a hydrogen atom, while alanine has a methyl side chain. The 'R' group in serine is CH - OH. Amongst other things, the side chains affect whether the amino acid is hydrophilic (attracted to water) or hydrophobic (repelled by water). If the side chain is polar , then the amino acid is hydrophilic, but if the side chain is non-polar then the amino acid is hydrophobic. Glycine and alanine both have non-polar side chains, while serine has a polar side chain.
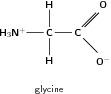
In an amino acid, the amino group acts as a base because the nitrogen atom has a pair of unpaired electrons which it can use to bond to a hydrogen ion. The amino group therefore attracts the hydrogen ion from the carboxyl group, and ends up having a charge of +1. The carboxyl group from which the hydrogen ion has been taken then has a charge of -1. The amino acid glycine can therefore also be represented as shown in the figure below.
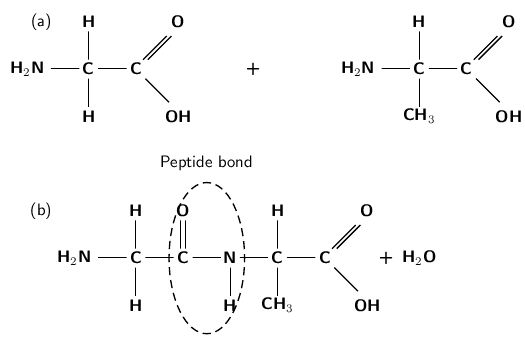
When two amino acid monomers are close together, they may be joined to each other by peptide bonds ( [link] ) to form a polypeptide chain. . The reaction is a condensation reaction. Polypeptides can vary in length from a few amino acids to a thousand or more. The polpeptide chains are then joined to each other in different ways to form a protein . It is the sequence of the amino acids in the polymer that gives a protein its particular properties.
The sequence of the amino acids in the chain is known as the protein's primary structure . As the chain grows in size, it begins to twist, curl and fold upon itself. The different parts of the polypeptide are held together by hydrogen bonds, which form between hydrogen atoms in one part of the chain and oxygen or nitrogen atoms in another part of the chain. This is known as the secondary structure of the protein. Sometimes, in this coiled helical structure, bonds may form between the side chains (R groups) of the amino acids. This results in even more irregular contortions of the protein. This is called the tertiary structure of the protein.
There are twenty different amino acids that exist in nature. All cells, both plant and animal, build their proteins from only twenty amino acids. At first, this seems like a very small number, especially considering the huge number of different proteins that exist. However, if you consider that most proteins are made up of polypeptide chains that contain at least 100 amino acids, you will start to realise the endless possible combinations of amino acids that are available.

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 12 physical science' conversation and receive update notifications?