| << Chapter < Page | Chapter >> Page > |
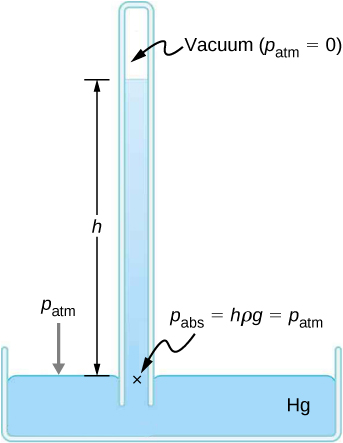
Weather forecasters closely monitor changes in atmospheric pressure (often reported as barometric pressure), as rising mercury typically signals improving weather and falling mercury indicates deteriorating weather. The barometer can also be used as an altimeter, since average atmospheric pressure varies with altitude. Mercury barometers and manometers are so common that units of mm Hg are often quoted for atmospheric pressure and blood pressures.
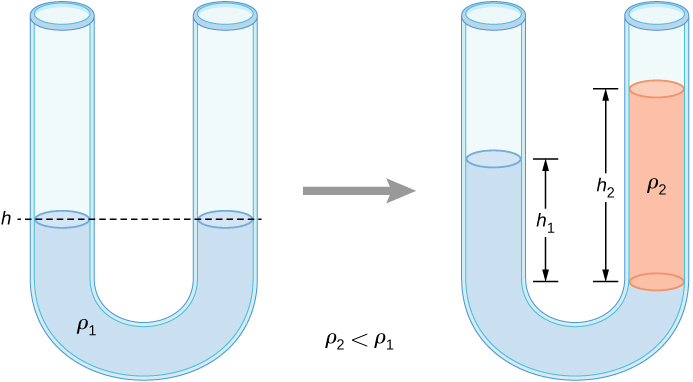
Hence,
This means that the difference in heights on the two sides of the U-tube is
The result makes sense if we set which gives If the two sides have the same density, they have the same height.
Check Your Understanding Mercury is a hazardous substance. Why do you suppose mercury is typically used in barometers instead of a safer fluid such as water?
The density of mercury is 13.6 times greater than the density of water. It takes approximately 76 cm (29.9 in.) of mercury to measure the pressure of the atmosphere, whereas it would take approximately 10 m (34 ft.) of water.
As stated earlier, the SI unit for pressure is the pascal (Pa), where
In addition to the pascal, many other units for pressure are in common use ( [link] ). In meteorology, atmospheric pressure is often described in the unit of millibars (mb), where
The millibar is a convenient unit for meteorologists because the average atmospheric pressure at sea level on Earth is . Using the equations derived when considering pressure at a depth in a fluid, pressure can also be measured as millimeters or inches of mercury. The pressure at the bottom of a 760-mm column of mercury at in a container where the top part is evacuated is equal to the atmospheric pressure. Thus, 760 mm Hg is also used in place of 1 atmosphere of pressure. In vacuum physics labs, scientists often use another unit called the torr, named after Torricelli, who, as we have just seen, invented the mercury manometer for measuring pressure. One torr is equal to a pressure of 1 mm Hg.
| Unit | Definition |
|---|---|
| SI unit: the Pascal | |
| English unit: pounds per square inch ( or psi) | |
| Other units of pressure | |
Explain why the fluid reaches equal levels on either side of a manometer if both sides are open to the atmosphere, even if the tubes are of different diameters.
The pressure of the atmosphere is due to the weight of the air above. The pressure, force per area, on the manometer will be the same at the same depth of the atmosphere.
Find the gauge and absolute pressures in the balloon and peanut jar shown in [link] , assuming the manometer connected to the balloon uses water and the manometer connected to the jar contains mercury. Express in units of centimeters of water for the balloon and millimeters of mercury for the jar, taking for each.
How tall must a water-filled manometer be to measure blood pressure as high as 300 mm Hg?
4.08 m
Assuming bicycle tires are perfectly flexible and support the weight of bicycle and rider by pressure alone, calculate the total area of the tires in contact with the ground if a bicycle and rider have a total mass of 80.0 kg, and the gauge pressure in the tires is .

Notification Switch
Would you like to follow the 'University physics volume 1' conversation and receive update notifications?