| << Chapter < Page | Chapter >> Page > |
Two objects of equal mass are moving with equal and opposite velocities when they collide. Can all the kinetic energy be lost in the collision?
Yes, all the kinetic energy can be lost if the two masses come to rest due to the collision (i.e., they stick together).
Describe a system for which momentum is conserved but mechanical energy is not. Now the reverse: Describe a system for which kinetic energy is conserved but momentum is not.
A 5.50-kg bowling ball moving at 9.00 m/s collides with a 0.850-kg bowling pin, which is scattered at an angle to the initial direction of the bowling ball and with a speed of 15.0 m/s.
a. 6.80 m/s, 5.33°; b. yes (calculate the ratio of the initial and final kinetic energies)
Ernest Rutherford (the first New Zealander to be awarded the Nobel Prize in Chemistry) demonstrated that nuclei were very small and dense by scattering helium-4 nuclei from gold-197 nuclei. The energy of the incoming helium nucleus was , and the masses of the helium and gold nuclei were and , respectively (note that their mass ratio is 4 to 197).
a. If a helium nucleus scatters to an angle of during an elastic collision with a gold nucleus, calculate the helium nucleus’s final speed and the final velocity (magnitude and direction) of the gold nucleus.
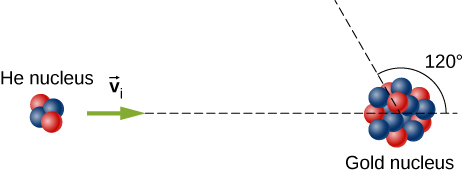
b. What is the final kinetic energy of the helium nucleus?
A 90.0-kg ice hockey player hits a 0.150-kg puck, giving the puck a velocity of 45.0 m/s. If both are initially at rest and if the ice is frictionless, how far does the player recoil in the time it takes the puck to reach the goal 15.0 m away?
2.5 cm
A 100-g firecracker is launched vertically into the air and explodes into two pieces at the peak of its trajectory. If a 72-g piece is projected horizontally to the left at 20 m/s, what is the speed and direction of the other piece?
In an elastic collision, a 400-kg bumper car collides directly from behind with a second, identical bumper car that is traveling in the same direction. The initial speed of the leading bumper car is 5.60 m/s and that of the trailing car is 6.00 m/s. Assuming that the mass of the drivers is much, much less than that of the bumper cars, what are their final speeds?
the speed of the leading bumper car is 6.00 m/s and that of the trailing bumper car is 5.60 m/s
Repeat the preceding problem if the mass of the leading bumper car is 30.0% greater than that of the trailing bumper car.
An alpha particle ( 4 He) undergoes an elastic collision with a stationary uranium nucleus ( 235 U). What percent of the kinetic energy of the alpha particle is transferred to the uranium nucleus? Assume the collision is one-dimensional.
6.6%
You are standing on a very slippery icy surface and throw a 1-kg football horizontally at a speed of 6.7 m/s. What is your velocity when you release the football? Assume your mass is 65 kg.
A 35-kg child sleds down a hill and then coasts along the flat section at the bottom, where a second 35-kg child jumps on the sled as it passes by her. If the speed of the sled is 3.5 m/s before the second child jumps on, what is its speed after she jumps on?
1.9 m/s
A boy sleds down a hill and onto a frictionless ice-covered lake at 10.0 m/s. In the middle of the lake is a 1000-kg boulder. When the sled crashes into the boulder, he is propelled over the boulder and continues sliding over the ice. If the boy’s mass is 40.0 kg and the sled’s mass is 2.50 kg, what is the speed of the sled and the boulder after the collision?

Notification Switch
Would you like to follow the 'University physics volume 1' conversation and receive update notifications?