| << Chapter < Page | Chapter >> Page > |
We need to compare the artery radius before and after the flow rate reduction. Note that we are given the diameter of the conduit, so we must divide by two to get the radius.
Doubling the length cuts the flow rate to one-half the original flow rate.
If the radius is decreased and all other variables remain constant, the volume flow rate decreases by a much larger factor.
Cutting the radius in half decreases the flow rate to one-sixteenth the original flow rate.
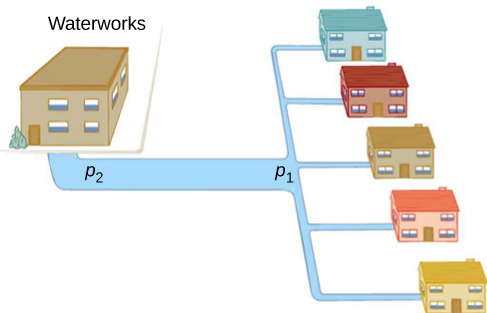
Water pressure in homes is sometimes lower than normal during times of heavy use, such as hot summer days. The drop in pressure occurs in the water main before it reaches individual homes. Let us consider flow through the water main as illustrated in [link] . We can understand why the pressure to the home drops during times of heavy use by rearranging the equation for flow rate:
In this case, is the pressure at the water works and R is the resistance of the water main. During times of heavy use, the flow rate Q is large. This means that must also be large. Thus must decrease. It is correct to think of flow and resistance as causing the pressure to drop from to . The equation is valid for both laminar and turbulent flows.

Notification Switch
Would you like to follow the 'University physics volume 1' conversation and receive update notifications?