| << Chapter < Page | Chapter >> Page > |
The properties of a conductor are consistent with the situations already discussed and can be used to analyze any conductor in electrostatic equilibrium. This can lead to some interesting new insights, such as described below.
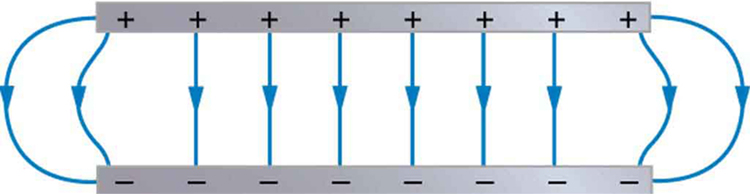
How can a very uniform electric field be created? Consider a system of two metal plates with opposite charges on them, as shown in [link] . The properties of conductors in electrostatic equilibrium indicate that the electric field between the plates will be uniform in strength and direction. Except near the edges, the excess charges distribute themselves uniformly, producing field lines that are uniformly spaced (hence uniform in strength) and perpendicular to the surfaces (hence uniform in direction, since the plates are flat). The edge effects are less important when the plates are close together.
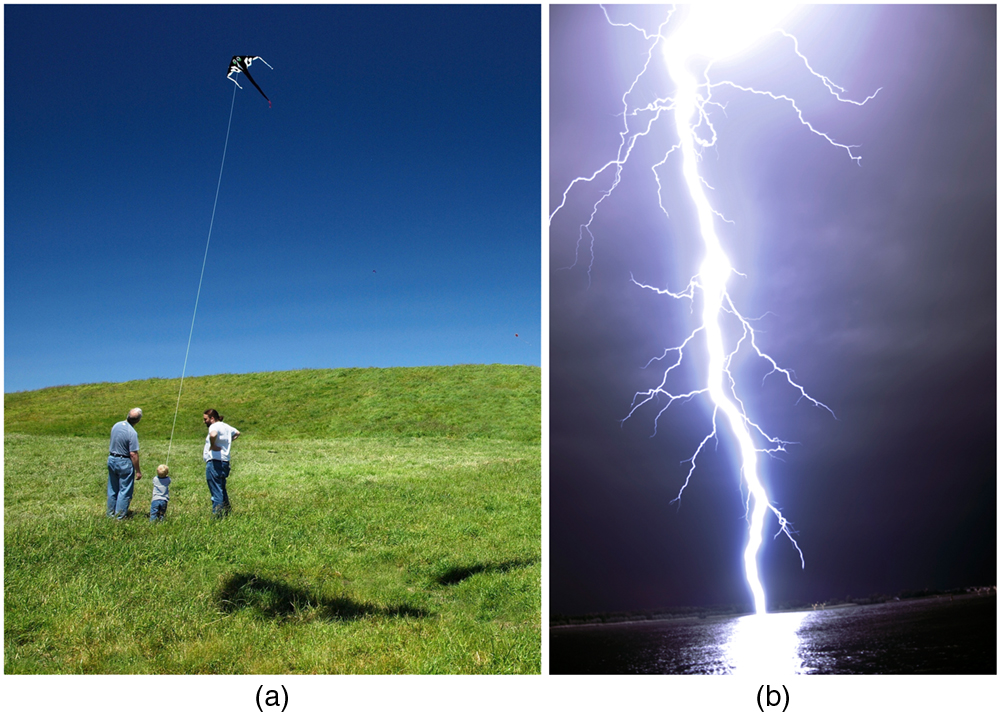
A near uniform electric field of approximately 150 N/C, directed downward, surrounds Earth, with the magnitude increasing slightly as we get closer to the surface. What causes the electric field? At around 100 km above the surface of Earth we have a layer of charged particles, called the ionosphere . The ionosphere is responsible for a range of phenomena including the electric field surrounding Earth. In fair weather the ionosphere is positive and the Earth largely negative, maintaining the electric field ( [link] (a)).
In storm conditions clouds form and localized electric fields can be larger and reversed in direction ( [link] (b)). The exact charge distributions depend on the local conditions, and variations of [link] (b) are possible.
If the electric field is sufficiently large, the insulating properties of the surrounding material break down and it becomes conducting. For air this occurs at around N/C. Air ionizes ions and electrons recombine, and we get discharge in the form of lightning sparks and corona discharge.
So far we have considered excess charges on a smooth, symmetrical conductor surface. What happens if a conductor has sharp corners or is pointed? Excess charges on a nonuniform conductor become concentrated at the sharpest points. Additionally, excess charge may move on or off the conductor at the sharpest points.

Notification Switch
Would you like to follow the 'College physics' conversation and receive update notifications?