| << Chapter < Page | Chapter >> Page > |
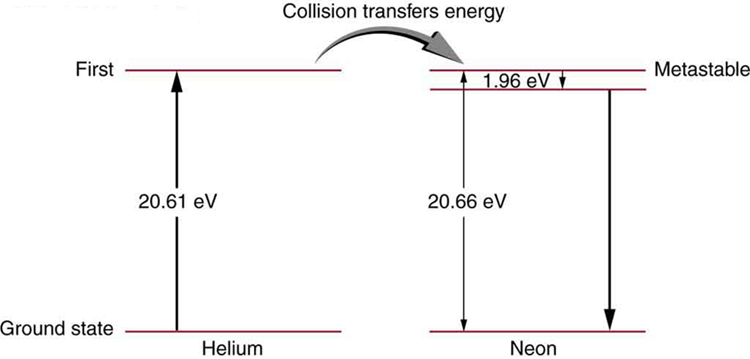
Lasers are constructed from many types of lasing materials, including gases, liquids, solids, and semiconductors. But all lasers are based on the existence of a metastable state or a phosphorescent material. Some lasers produce continuous output; others are pulsed in bursts as brief as . Some laser outputs are fantastically powerful—some greater than —but the more common, everyday lasers produce something on the order of . The helium-neon laser that produces a familiar red light is very common. [link] shows the energy levels of helium and neon, a pair of noble gases that work well together. An electrical discharge is passed through a helium-neon gas mixture in which the number of atoms of helium is ten times that of neon. The first excited state of helium is metastable and, thus, stores energy. This energy is easily transferred by collision to neon atoms, because they have an excited state at nearly the same energy as that in helium. That state in neon is also metastable, and this is the one that produces the laser output. (The most likely transition is to the nearby state, producing 1.96 eV photons, which have a wavelength of 633 nm and appear red.) A population inversion can be produced in neon, because there are so many more helium atoms and these put energy into the neon. Helium-neon lasers often have continuous output, because the population inversion can be maintained even while lasing occurs. Probably the most common lasers in use today, including the common laser pointer, are semiconductor or diode lasers, made of silicon. Here, energy is pumped into the material by passing a current in the device to excite the electrons. Special coatings on the ends and fine cleavings of the semiconductor material allow light to bounce back and forth and a tiny fraction to emerge as laser light. Diode lasers can usually run continually and produce outputs in the milliwatt range.
There are many medical applications of lasers. Lasers have the advantage that they can be focused to a small spot. They also have a well-defined wavelength. Many types of lasers are available today that provide wavelengths from the ultraviolet to the infrared. This is important, as one needs to be able to select a wavelength that will be preferentially absorbed by the material of interest. Objects appear a certain color because they absorb all other visible colors incident upon them. What wavelengths are absorbed depends upon the energy spacing between electron orbitals in that molecule. Unlike the hydrogen atom, biological molecules are complex and have a variety of absorption wavelengths or lines. But these can be determined and used in the selection of a laser with the appropriate wavelength. Water is transparent to the visible spectrum but will absorb light in the UV and IR regions. Blood (hemoglobin) strongly reflects red but absorbs most strongly in the UV.

Notification Switch
Would you like to follow the 'College physics' conversation and receive update notifications?