| << Chapter < Page | Chapter >> Page > |
The tendency of a fluid to be raised or suppressed in a narrow tube, or capillary tube, is called capillary action.
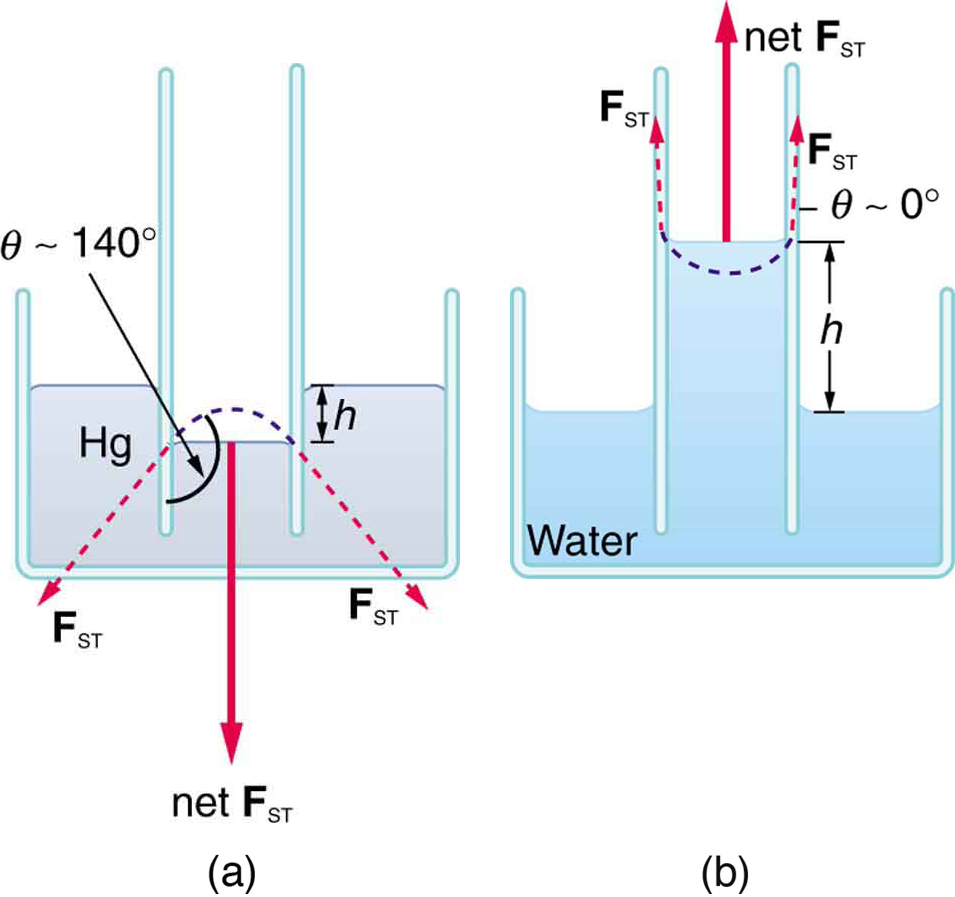
If a capillary tube is placed vertically into a liquid, as shown in [link] , capillary action will raise or suppress the liquid inside the tube depending on the combination of substances. The actual effect depends on the relative strength of the cohesive and adhesive forces and, thus, the contact angle given in the table. If is less than , then the fluid will be raised; if is greater than , it will be suppressed. Mercury, for example, has a very large surface tension and a large contact angle with glass. When placed in a tube, the surface of a column of mercury curves downward, somewhat like a drop. The curved surface of a fluid in a tube is called a meniscus . The tendency of surface tension is always to reduce the surface area. Surface tension thus flattens the curved liquid surface in a capillary tube. This results in a downward force in mercury and an upward force in water, as seen in [link] .
| Interface | Contact angle Θ |
|---|---|
| Mercury–glass | |
| Water–glass | |
| Water–paraffin | |
| Water–silver | |
| Organic liquids (most)–glass | |
| Ethyl alcohol–glass | |
| Kerosene–glass |
Capillary action can move liquids horizontally over very large distances, but the height to which it can raise or suppress a liquid in a tube is limited by its weight. It can be shown that this height is given by
If we look at the different factors in this expression, we might see how it makes good sense. The height is directly proportional to the surface tension , which is its direct cause. Furthermore, the height is inversely proportional to tube radius—the smaller the radius , the higher the fluid can be raised, since a smaller tube holds less mass. The height is also inversely proportional to fluid density , since a larger density means a greater mass in the same volume. (See [link] .)
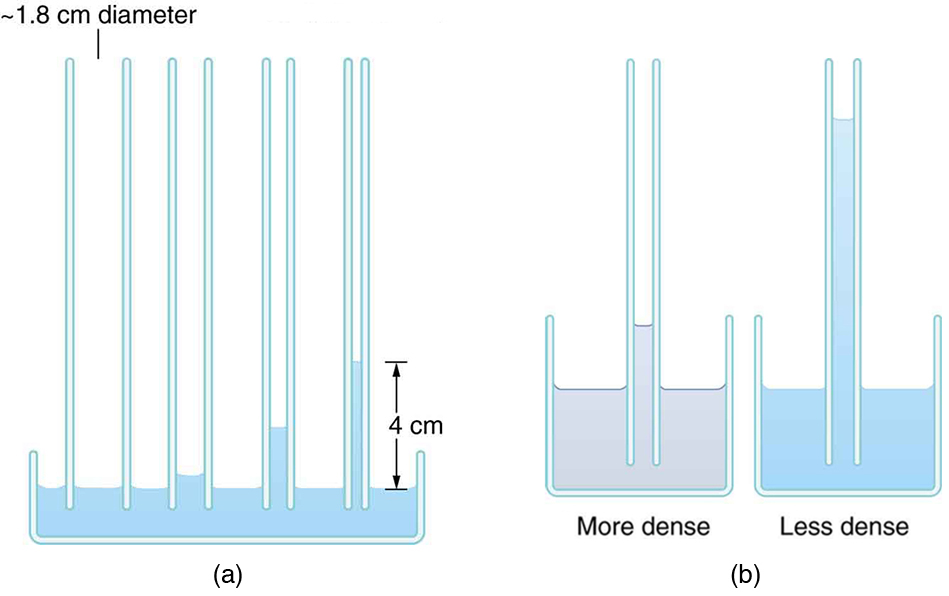
Can capillary action be solely responsible for sap rising in trees? To answer this question, calculate the radius of a capillary tube that would raise sap 100 m to the top of a giant redwood, assuming that sap’s density is , its contact angle is zero, and its surface tension is the same as that of water at .
Strategy
The height to which a liquid will rise as a result of capillary action is given by , and every quantity is known except for .
Solution
Solving for and substituting known values produces
Discussion
This result is unreasonable. Sap in trees moves through the xylem , which forms tubes with radii as small as . This value is about 180 times as large as the radius found necessary here to raise sap . This means that capillary action alone cannot be solely responsible for sap getting to the tops of trees.

Notification Switch
Would you like to follow the 'College physics' conversation and receive update notifications?