| << Chapter < Page | Chapter >> Page > |
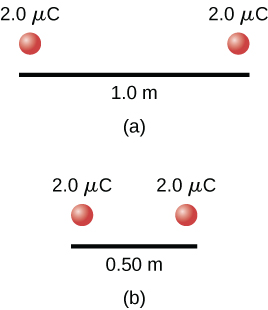
Consider a system consisting of two positive point charges, each 2.0 µC, placed 1.0 m away from each other. We can calculate the potential (i.e. internal) energy of this configuration by computing the potential due to one of the charges, and then calculating the potential energy of the second charge in the potential of the first. Applying Equations (19.38) and (19.2) gives us a potential energy of 3.6×10 -2 J. If we move the charges closer to each other, say, to 0.50 m apart, the potential energy doubles. Note that, to create this second case, some outside force would have had to do work on this system to change the configuration, and hence it was not a closed system. However, because the electric force is conservative, we can use the work-energy theorem to state that, since there was no change in kinetic energy, all of the work done went into increasing the internal energy of the system. Also note that if the point charges had different signs they would be attracted to each other, so they would be capable of doing work on an outside system when the distance between them decreased. As work is done on the outside system, the internal energy in the two-charge system decreases.
The energy per electron is very small in macroscopic situations like that in the previous example—a tiny fraction of a joule. But on a submicroscopic scale, such energy per particle (electron, proton, or ion) can be of great importance. For example, even a tiny fraction of a joule can be great enough for these particles to destroy organic molecules and harm living tissue. The particle may do its damage by direct collision, or it may create harmful x rays, which can also inflict damage. It is useful to have an energy unit related to submicroscopic effects. [link] shows a situation related to the definition of such an energy unit. An electron is accelerated between two charged metal plates as it might be in an old-model television tube or oscilloscope. The electron is given kinetic energy that is later converted to another form—light in the television tube, for example. (Note that downhill for the electron is uphill for a positive charge.) Since energy is related to voltage by we can think of the joule as a coulomb-volt.
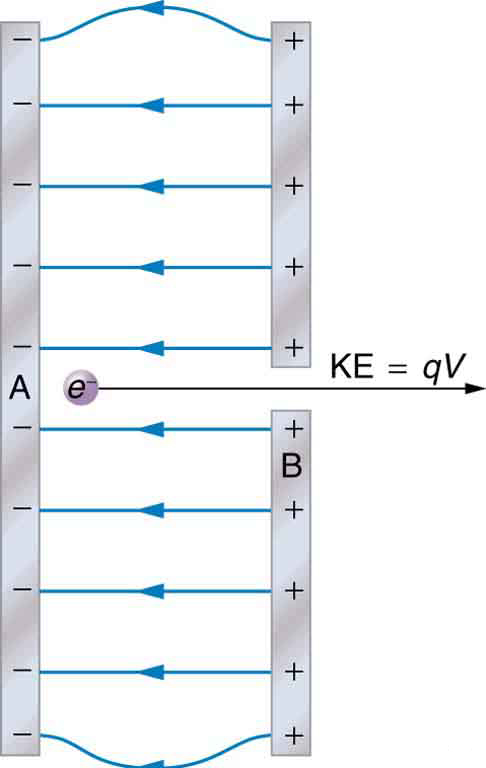
On the submicroscopic scale, it is more convenient to define an energy unit called the electron volt (eV), which is the energy given to a fundamental charge accelerated through a potential difference of 1 V. In equation form,
On the submicroscopic scale, it is more convenient to define an energy unit called the electron volt (eV), which is the energy given to a fundamental charge accelerated through a potential difference of 1 V . In equation form,

Notification Switch
Would you like to follow the 'College physics for ap® courses' conversation and receive update notifications?