| << Chapter < Page | Chapter >> Page > |
By the end of this section, you will be able to:
The information presented in this section supports the following AP® learning objectives and science practices:
In Work, Energy, and Energy Resources , we defined work as force times distance and learned that work done on an object changes its kinetic energy. We also saw in Temperature, Kinetic Theory, and the Gas Laws that temperature is proportional to the (average) kinetic energy of atoms and molecules. We say that a thermal system has a certain internal energy: its internal energy is higher if the temperature is higher. If two objects at different temperatures are brought in contact with each other, energy is transferred from the hotter to the colder object until equilibrium is reached and the bodies reach thermal equilibrium (i.e., they are at the same temperature). No work is done by either object, because no force acts through a distance. The transfer of energy is caused by the temperature difference, and ceases once the temperatures are equal. These observations lead to the following definition of heat : Heat is the spontaneous transfer of energy due to a temperature difference.
As noted in Temperature, Kinetic Theory, and the Gas Laws , heat is often confused with temperature. For example, we may say the heat was unbearable, when we actually mean that the temperature was high. Heat is a form of energy, whereas temperature is not. The misconception arises because we are sensitive to the flow of heat, rather than the temperature.
Owing to the fact that heat is a form of energy, it has the SI unit of joule (J). The calorie (cal) is a common unit of energy, defined as the energy needed to change the temperature of 1.00 g of water by —specifically, between and , since there is a slight temperature dependence. Perhaps the most common unit of heat is the kilocalorie (kcal), which is the energy needed to change the temperature of 1.00 kg of water by . Since mass is most often specified in kilograms, kilocalorie is commonly used. Food calories (given the notation Cal, and sometimes called “big calorie”) are actually kilocalories ( ), a fact not easily determined from package labeling.
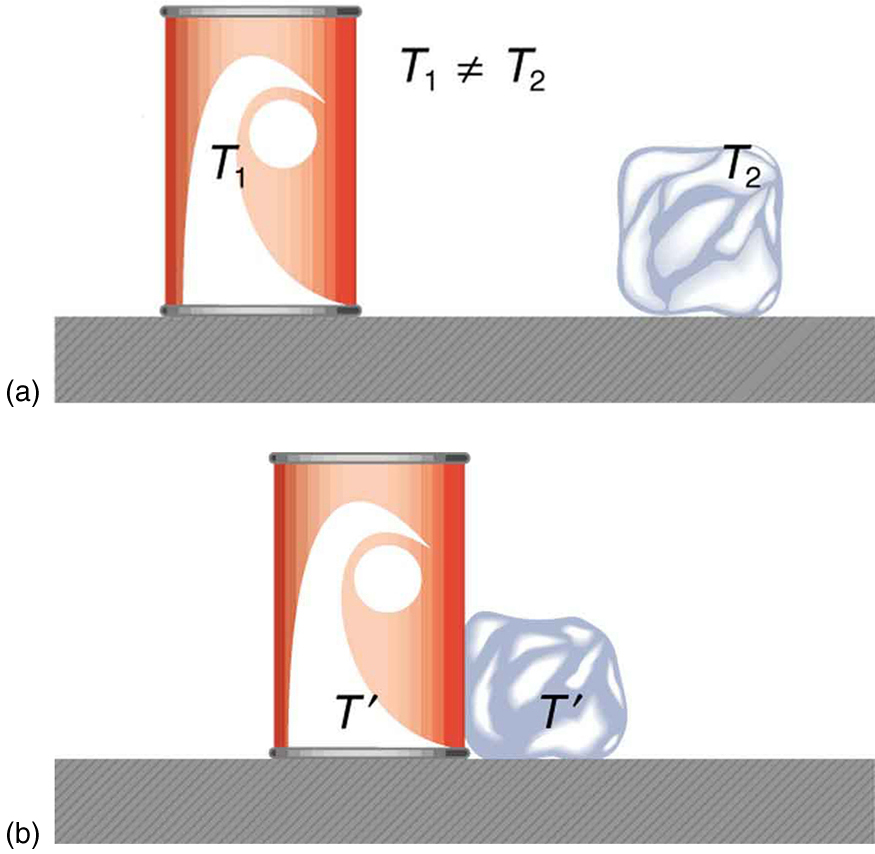
What is observed as a change in temperature of two macroscopic objects in contact, such as a warm can of liquid and an ice cube, consists of the transfer of kinetic energy from particles (atoms or molecules) with greater kinetic energy to those with lower kinetic energy. In this respect, the process can be viewed in terms of collisions, as described through classical mechanics. Consider the particles in two substances at different temperatures. The particles of each substance move with a range of speeds that are distributed around a mean value, . The temperature of each substance is defined in terms of the average kinetic energy of its particles, . The simplest mathematical description of this is for an ideal gas, and is given by the following equation:
where k is Boltzmann’s constant ( ). The equations for non-ideal gases, liquids, and solids are more complicated, but the general relation between the kinetic energies of the particles and the overall temperature of the substance still holds: the particles in the substance with the higher temperature have greater average kinetic energies than do the particles of a substance with a lower temperature.
When the two substances are in thermal contact, the particles of both substances can collide with each other. In the vast majority of collisions, a particle with greater kinetic energy will transfer some of its energy to a particle with lower kinetic energy. By giving up this energy, the average kinetic energy of this particle is reduced, and therefore, the temperature of the substance associated with that particle decreases slightly. Similarly, the average kinetic energy of the particle in the second substance increases through the collision, causing that substance’s temperature to increase by a minuscule amount. In this way, through a vast number of particle collisions, thermal energy is transferred macroscopically from the substance with greater temperature (that is, greater internal energy) to the substance with lower temperature (lower internal energy).
Macroscopically, heat appears to transfer thermal energy spontaneously in only one direction. When interpreted at the microscopic level, the transfer of kinetic energy between particles occurs in both directions. This is because some of the particles in the low-temperature substance have higher kinetic energies than the particles in the high-temperature substance, so that some of the energy transfer is in the direction from the lower temperature substance to the higher temperature substance. However, much more of the energy is transferred in the other direction. When thermal equilibrium is reached, the energy transfer in either direction is, on average, the same, so that there is no further change in the internal energy, or temperature, of either substance.

Notification Switch
Would you like to follow the 'College physics for ap® courses' conversation and receive update notifications?