| << Chapter < Page | Chapter >> Page > |
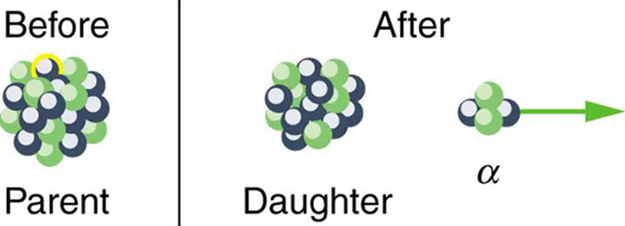
Note that the daughters of decay shown in [link] always have two fewer protons and two fewer neutrons than the parent. This seems reasonable, since we know that decay is the emission of a nucleus, which has two protons and two neutrons. The daughters of decay have one less neutron and one more proton than their parent. Beta decay is a little more subtle, as we shall see. No decays are shown in the figure, because they do not produce a daughter that differs from the parent.
In alpha decay , a nucleus simply breaks away from the parent nucleus, leaving a daughter with two fewer protons and two fewer neutrons than the parent (see [link] ). One example of decay is shown in [link] for . Another nuclide that undergoes decay is . The decay equations for these two nuclides are
and
If you examine the periodic table of the elements, you will find that Th has , two fewer than U, which has . Similarly, in the second decay equation , we see that U has two fewer protons than Pu, which has . The general rule for decay is best written in the format . If a certain nuclide is known to decay (generally this information must be looked up in a table of isotopes, such as in [link] ), its decay equation is
where Y is the nuclide that has two fewer protons than X, such as Th having two fewer than U. So if you were told that decays and were asked to write the complete decay equation, you would first look up which element has two fewer protons (an atomic number two lower) and find that this is uranium. Then since four nucleons have broken away from the original 239, its atomic mass would be 235.
It is instructive to examine conservation laws related to decay. You can see from the equation that total charge is conserved. Linear and angular momentum are conserved, too. Although conserved angular momentum is not of great consequence in this type of decay, conservation of linear momentum has interesting consequences. If the nucleus is at rest when it decays, its momentum is zero. In that case, the fragments must fly in opposite directions with equal-magnitude momenta so that total momentum remains zero. This results in the particle carrying away most of the energy, as a bullet from a heavy rifle carries away most of the energy of the powder burned to shoot it. Total mass–energy is also conserved: the energy produced in the decay comes from conversion of a fraction of the original mass. As discussed in [link] , the general relationship is
Here, is the nuclear reaction energy (the reaction can be nuclear decay or any other reaction), and is the difference in mass between initial and final products. When the final products have less total mass, is positive, and the reaction releases energy (is exothermic). When the products have greater total mass, the reaction is endothermic ( is negative) and must be induced with an energy input. For decay to be spontaneous, the decay products must have smaller mass than the parent.

Notification Switch
Would you like to follow the 'College physics for ap® courses' conversation and receive update notifications?