| << Chapter < Page | Chapter >> Page > |
The observed hydrogen-spectrum wavelengths can be calculated using the following formula:
where is the wavelength of the emitted EM radiation and is the Rydberg constant , determined by the experiment to be
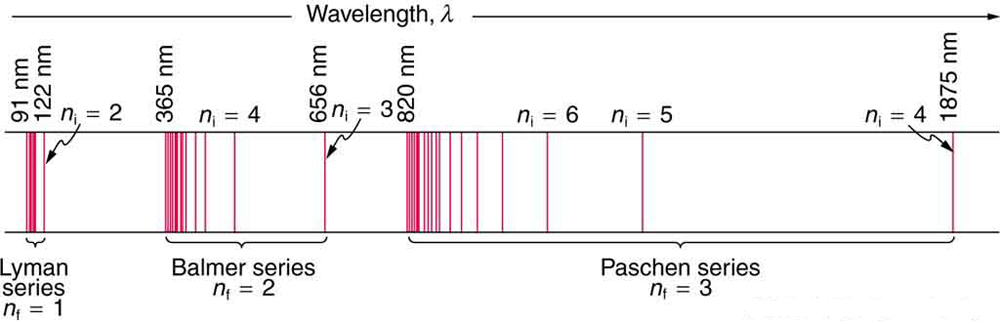
The constant is a positive integer associated with a specific series. For the Lyman series, ; for the Balmer series, ; for the Paschen series, ; and so on. The Lyman series is entirely in the UV, while part of the Balmer series is visible with the remainder UV. The Paschen series and all the rest are entirely IR. There are apparently an unlimited number of series, although they lie progressively farther into the infrared and become difficult to observe as increases. The constant is a positive integer, but it must be greater than . Thus, for the Balmer series, and . Note that can approach infinity. While the formula in the wavelengths equation was just a recipe designed to fit data and was not based on physical principles, it did imply a deeper meaning. Balmer first devised the formula for his series alone, and it was later found to describe all the other series by using different values of . Bohr was the first to comprehend the deeper meaning. Again, we see the interplay between experiment and theory in physics. Experimentally, the spectra were well established, an equation was found to fit the experimental data, but the theoretical foundation was missing.
What is the distance between the slits of a grating that produces a first-order maximum for the second Balmer line at an angle of ?
Strategy and Concept
For an Integrated Concept problem, we must first identify the physical principles involved. In this example, we need to know (a) the wavelength of light as well as (b) conditions for an interference maximum for the pattern from a double slit. Part (a) deals with a topic of the present chapter, while part (b) considers the wave interference material of Wave Optics .
Solution for (a)
Hydrogen spectrum wavelength . The Balmer series requires that . The first line in the series is taken to be for , and so the second would have .
The calculation is a straightforward application of the wavelength equation. Entering the determined values for and yields
Inverting to find gives
Discussion for (a)
This is indeed the experimentally observed wavelength, corresponding to the second (blue-green) line in the Balmer series. More impressive is the fact that the same simple recipe predicts all of the hydrogen spectrum lines, including new ones observed in subsequent experiments. What is nature telling us?
Solution for (b)
Double-slit interference ( Wave Optics ). To obtain constructive interference for a double slit, the path length difference from two slits must be an integral multiple of the wavelength. This condition was expressed by the equation
where is the distance between slits and is the angle from the original direction of the beam. The number is the order of the interference; in this example. Solving for and entering known values yields
Discussion for (b)
This number is similar to those used in the interference examples of Introduction to Quantum Physics (and is close to the spacing between slits in commonly used diffraction glasses).

Notification Switch
Would you like to follow the 'College physics' conversation and receive update notifications?