| << Chapter < Page | Chapter >> Page > |
In studying knots, mathematicians often make the assumption that all knots under consideration are smooth. Yet knots that appear in nature are often rigid. A stick knot, or polygonal knot, is a knot composed of line segments attached at their edges. Much work has been done over the last few decades towards determining the minimum number of sticks necessary to construct a given knot in . In this PFUG we ask the same question when restrictions are placed on the way the sticks may lay in . That is, we seek to determine the minimum number of Legendrian sticks necessary to construct a given Legendrian knot.
Definition 2.1 A contact structure on assigns to each point a plane . The standard contact structure assigns to the point the plane
where we orient via the Right Hand Rule.
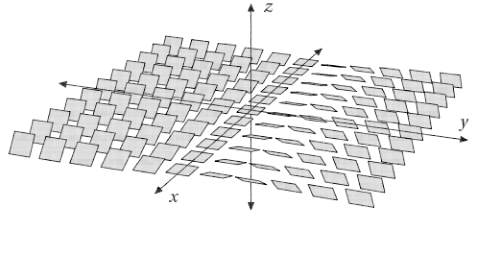
Definition 2.2 A knot in is the image of an embedding . A Legendrian knot in is a knot such that
for all .
Observe that if parametrizes a knot then for to be Legendrian in the standard contact structure, must be orthogonal to
Whenever we draw a knot in we are actually drawing a projection of the knot in some plane with labeled crossings. We use the front and Lagrangian projections to draw Legendrian knots.

Definition 2.3 Let such that . The front projection of , denoted , is the image of under . If above parametrizes then parametrizes .
We see from the identity that the front projection of a Legendrian knot cannot have vertical tangencies, and at each crossing the slope of the over-crossing segment must be less than the slope of the under-crossing segment.
Definition 2.4 Let such that . The Lagrangian projection of is the image of under and is denoted . As with the front projection, is parametrized by .
Definition 3.1 Two Legendrian knots are Legendrian isotopic if there is an isotopy between them such that parametrizes a Legendrian knot for each .
Theorem 3.2 Two front projections represent Legendrian isotopic Legendrian knots if and only if they are related by regular isotopy and a finite sequence of the moves below.

Theorem 3.3 If two Lagrangian projections represent Legendrian isotopic Legendrian knots then they are related by a sequence of the moves and , below.

Notification Switch
Would you like to follow the 'The art of the pfug' conversation and receive update notifications?