| << Chapter < Page | Chapter >> Page > |
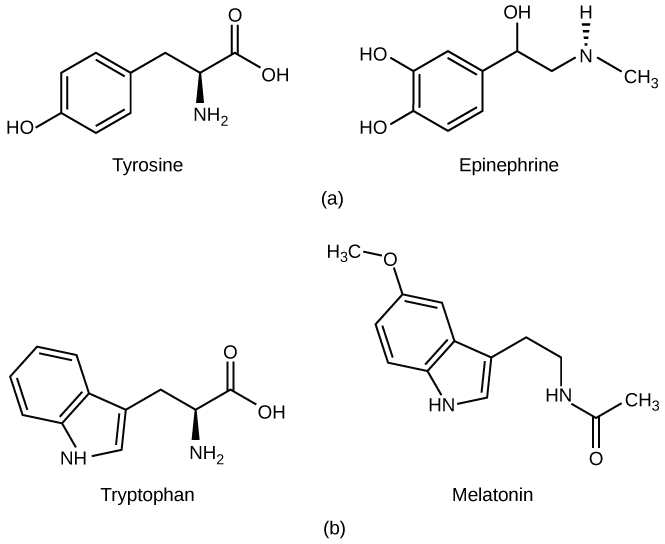
The peptide hormones include polypeptides as well as several relatively small molecules that are derived from the amino acids tyrosine and tryptophan, shown in [link] . Examples of amino acid-derived hormones include epinephrine and norepinephrine, which are synthesized in the medulla of the adrenal glands, and thyroxine, which is produced by the thyroid gland. The pineal gland in the brain makes and secretes melatonin which regulates sleep cycles.
Other peptide hormones are polypeptides (chains of amino acids linked by peptide bonds). These hormones include molecules that are quite short polypeptide chains, such as antidiuretic hormone (9 amino acids) and oxytocin (also 9 amino acids), both of which are produced in the brain and released into the blood in the posterior pituitary gland. This class also includes small proteins, like the growth hormones (approx 190 amino acids( produced by the pituitary, and large glycoproteins such as follicle-stimulating hormone (a complex of 2 different polypeptides, each about 100 amino acids in length), produced by the pituitary. [link] illustrates these peptide hormones.
Secreted peptides like insulin are stored within vesicles in the cells that synthesize them. They are then released in response to stimuli such as high blood glucose levels in the case of insulin. Amino acid-derived and polypeptide hormones are water-soluble. Therefore these hormones cannot cross the plasma membranes of cells; their receptors are found on the surface of the target cells.
Hormones mediate changes in target cells after binding to specific hormone receptors . In this way, even though hormones circulate throughout the body and come into contact with many different cell types, they only affect cells that possess the necessary receptors. Receptors for a specific hormone may be found on many different cells or may be limited to a small number of specialized cells. For example, thyroid hormones act on many different tissue types, stimulating metabolic activity throughout the body; testosterone receptors are found in relatively few cell types. Cells can have many receptors for the same hormone but often also possess receptors for different types of hormones. The number of receptors that respond to a hormone determines the cell’s sensitivity to that hormone, and the resulting cellular response.
Receptor binding alters cellular activity and results in an increase or decrease in normal body processes. Depending on the location of the protein receptor on the target cell and the chemical structure of the hormone, hormones can mediate changes directly by binding to intracellular hormone receptors and modulating gene transcription, or indirectly by binding to cell surface receptors and stimulating signaling pathways.

Notification Switch
Would you like to follow the 'Principles of biology' conversation and receive update notifications?