| << Chapter < Page | Chapter >> Page > |
ATP is a highly unstable molecule. Unless quickly used to perform work, ATP spontaneously dissociates into ADP + P i , and the free energy released during this process is lost as heat. The second question posed above, that is, how the energy released by ATP hydrolysis is used to perform work inside the cell, depends on a strategy called energy coupling. Cells couple the exergonic reaction of ATP hydrolysis with endergonic reactions, allowing them to proceed. One example of energy coupling using ATP involves a transmembrane ion pump that is extremely important for cellular function. This sodium-potassium pump (Na + /K + pump) drives sodium out of the cell and potassium into the cell ( [link] ). A large percentage of a cell’s ATP is spent powering this pump, because cellular processes bring a great deal of sodium into the cell and potassium out of the cell. The pump works constantly to stabilize cellular concentrations of sodium and potassium. In order for the pump to turn one cycle (exporting three Na+ ions and importing two K + ions), one molecule of ATP must be hydrolyzed. When ATP is hydrolyzed, its gamma phosphate doesn’t simply float away, but is actually transferred onto the pump protein. This process of a phosphate group binding to a molecule is called phosphorylation. As with most cases of ATP hydrolysis, a phosphate from ATP is transferred onto another molecule. In a phosphorylated state, the Na + /K + pump has more free energy and is triggered to undergo a conformational change. This change allows it to release Na + to the outside of the cell. It then binds extracellular K + , which, through another conformational change, causes the phosphate to detach from the pump. This release of phosphate triggers the K + to be released to the inside of the cell. Essentially, the energy released from the hydrolysis of ATP is coupled with the energy required to power the pump and transport Na + and K + ions. ATP performs cellular work using this basic form of energy coupling through phosphorylation.
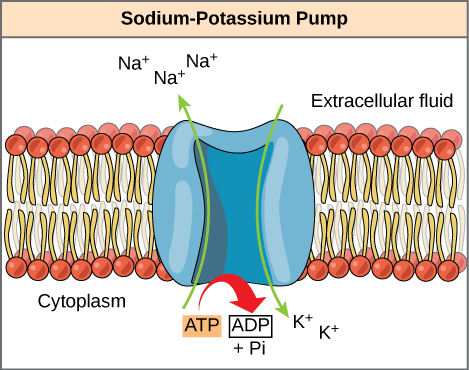
The hydrolysis of one ATP molecule releases 7.3 kcal/mol of energy (∆G = −7.3 kcal/mol of energy). If it takes 2.1 kcal/mol of energy to move one Na + across the membrane (∆G = +2.1 kcal/mol of energy), how many sodium ions could be moved by the hydrolysis of one ATP molecule?
Often during cellular metabolic reactions, such as the synthesis and breakdown of nutrients, certain molecules must be altered slightly in their conformation to become substrates for the next step in the reaction series. One example is during the very first steps of cellular respiration, when a molecule of the sugar glucose is broken down in the process of glycolysis. In the first step of this process, ATP is required for the phosphorylation of glucose, creating a high-energy but unstable intermediate. This phosphorylation reaction powers a conformational change that allows the phosphorylated glucose molecule to be converted to the phosphorylated sugar fructose. Fructose is a necessary intermediate for glycolysis to move forward. Here, the exergonic reaction of ATP hydrolysis is coupled with the endergonic reaction of converting glucose into a phosphorylated intermediate in the pathway. Once again, the energy released by breaking a phosphate bond within ATP was used for the phosphorylation of another molecule, creating an unstable intermediate and powering an important conformational change.
See an interactive animation of the ATP-producing glycolysis process at this site .
ATP is the primary energy-supplying molecule for living cells. ATP is made up of a nucleotide, a five-carbon sugar, and three phosphate groups. The bonds that connect the phosphates (phosphoanhydride bonds) have high-energy content. The energy released from the hydrolysis of ATP into ADP + P i is used to perform cellular work. Cells use ATP to perform work by coupling the exergonic reaction of ATP hydrolysis with endergonic reactions. ATP donates its phosphate group to another molecule via a process known as phosphorylation. The phosphorylated molecule is at a higher-energy state and is less stable than its unphosphorylated form, and this added energy from the addition of the phosphate allows the molecule to undergo its endergonic reaction.
[link] The hydrolysis of one ATP molecule releases 7.3 kcal/mol of energy (∆G = −7.3 kcal/mol of energy). If it takes 2.1 kcal/mol of energy to move one Na + across the membrane (∆G = +2.1 kcal/mol of energy), how many sodium ions could be moved by the hydrolysis of one ATP molecule?
[link] Three sodium ions could be moved by the hydrolysis of one ATP molecule. The ∆G of the coupled reaction must be negative. Movement of three sodium ions across the membrane will take 6.3 kcal of energy (2.1 kcal × 3 Na + ions = 6.3 kcal). Hydrolysis of ATP provides 7.3 kcal of energy, more than enough to power this reaction. Movement of four sodium ions across the membrane, however, would require 8.4 kcal of energy, more than one ATP molecule can provide.

Notification Switch
Would you like to follow the 'Cell biology' conversation and receive update notifications?