Ph changes in buffered and unbuffered solutions
Acetate buffers are used in biochemical studies of enzymes and other chemical components of cells to prevent pH changes that might change the biochemical activity of these compounds.
(a) Calculate the pH of an acetate buffer that is a mixture with 0.10
M acetic acid and 0.10
M sodium acetate.
Solution
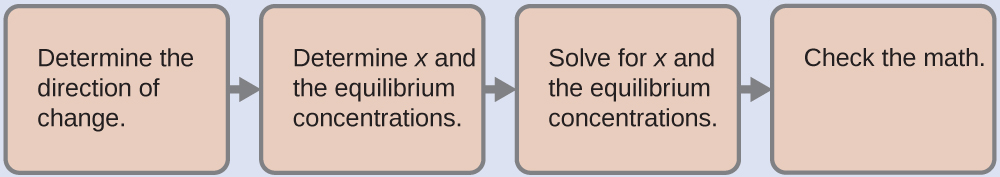
To determine the pH of the buffer solution we use a typical equilibrium calculation (as illustrated in earlier Examples):
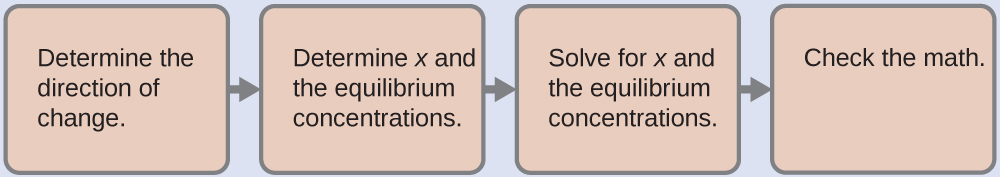
-
Determine the direction of change. The equilibrium in a mixture of H
3 O
+ ,
and CH
3 CO
2 H is:
The equilibrium constant for CH
3 CO
2 H is not given, so we look it up in
Appendix H :
K
a = 1.8
10
−5 . With [CH
3 CO
2 H] =
= 0.10
M and [H
3 O
+ ] = ~0
M , the reaction shifts to the right to form H
3 O
+ .
-
Determine x
and equilibrium concentrations . A table of changes and concentrations follows:
![This table has two main columns and four rows. The first row for the first column does not have a heading and then has the following in the first column: Initial concentration ( M ), Change ( M ), Equilibrium ( M ). The second column has the header of “[ C H subscript 3 C O subscript 2 H ] [ H subscript 2 O ] equilibrium arrow H subscript 3 O superscript plus sign [ C H subscript 3 C O subscript 2 superscript negative sign ].” Under the second column is a subgroup of four columns and three rows. The first column has the following: 0.10, negative x, 0.10 minus sign x. The second column is blank. The third column has the following: approximately 0, x, x. The fourth column has the following: 0.10, x, 0.10 plus sign x.](/ocw/mirror/col11830_1.13_complete/m51123/CNX_Chem_14_06_ICETable16_img.jpg)
-
Solve for x and the equilibrium concentrations. We find:
and
Thus:
-
Check the work . If we calculate all calculated equilibrium concentrations, we find that the equilibrium value of the reaction coefficient,
Q =
K
a .
(b) Calculate the pH after 1.0 mL of 0.10
M NaOH is added to 100 mL of this buffer, giving a solution with a volume of 101 mL.
First, we calculate the concentrations of an intermediate mixture resulting from the complete reaction between the acid in the buffer and the added base. Then we determine the concentrations of the mixture at the new equilibrium:
![Eight tan rectangles are shown in four columns of two rectangles each that are connected with right pointing arrows. The first rectangle in the upper left is labeled “Volume of N a O H solution.” An arrow points right to a second rectangle labeled “Moles of N a O H added.” A second arrow points right to a third rectangle labeled “Additional moles of N a C H subscript 3 C O subscript 2.” Just beneath the first rectangle in the upper left is a rectangle labeled “Volume of buffer solution.” An arrow points right to another rectangle labeled “Initial moles of C H subscript 3 C O subscript 2 H.” This rectangle points to the same third rectangle, which is labeled “ Additional moles of N a C H subscript 3 C O subscript 2.” An arrow points right to a rectangle labeled “ Unreacted moles of C H subscript 3 C O subscript 2 H.” An arrow points from this rectangle to a rectangle below labeled “[ C H subscript 3 C O subscript 2 H ].” An arrow extends below the “Additional moles of N a C H subscript 3 C O subscript 2” rectangle to a rectangle labeled “[ C H subscript 3 C O subscript 2 ].” This rectangle points right to the rectangle labeled “[ C H subscript 3 C O subscript 2 H ].”](/ocw/mirror/col11830_1.13_complete/m51123/CNX_Chem_14_06_steps2_img.jpg)
-
Determine the moles of NaOH. One milliliter (0.0010 L) of 0.10
M NaOH contains:
-
Determine the moles of CH
2 CO
2 H. Before reaction, 0.100 L of the buffer solution contains:
-
Solve for the amount of NaCH
3 CO
2 produced. The 1.0
10
−4 mol of NaOH neutralizes 1.0
10
−4 mol of CH
3 CO
2 H, leaving:
and producing 1.0
10
−4 mol of NaCH
3 CO
2 . This makes a total of:
-
Find the molarity of the products. After reaction, CH
3 CO
2 H and NaCH
3 CO
2 are contained in 101 mL of the intermediate solution, so:
Now we calculate the pH after the intermediate solution, which is 0.098
M in CH
3 CO
2 H and 0.100
M in NaCH
3 CO
2 , comes to equilibrium. The calculation is very similar to that in part (a) of this example:
This series of calculations gives a pH = 4.75. Thus the addition of the base barely changes the pH of the solution (
[link] ).
(c) For comparison, calculate the pH after 1.0 mL of 0.10
M NaOH is added to 100 mL of a solution of an unbuffered solution with a pH of 4.74 (a 1.8
10
−5 -
M solution of HCl). The volume of the final solution is 101 mL.
Solution
This 1.8
10
−5 -
M solution of HCl has the same hydronium ion concentration as the 0.10-
M solution of acetic acid-sodium acetate buffer described in part (a) of this example. The solution contains:
As shown in part (b), 1 mL of 0.10
M NaOH contains 1.0
10
−4 mol of NaOH. When the NaOH and HCl solutions are mixed, the HCl is the limiting reagent in the reaction. All of the HCl reacts, and the amount of NaOH that remains is:

![This table has two main columns and four rows. The first row for the first column does not have a heading and then has the following in the first column: Initial concentration ( M ), Change ( M ), Equilibrium ( M ). The second column has the header of “[ C H subscript 3 C O subscript 2 H ] [ H subscript 2 O ] equilibrium arrow H subscript 3 O superscript plus sign [ C H subscript 3 C O subscript 2 superscript negative sign ].” Under the second column is a subgroup of four columns and three rows. The first column has the following: 0.10, negative x, 0.10 minus sign x. The second column is blank. The third column has the following: approximately 0, x, x. The fourth column has the following: 0.10, x, 0.10 plus sign x.](/ocw/mirror/col11830_1.13_complete/m51123/CNX_Chem_14_06_ICETable16_img.jpg)
![Eight tan rectangles are shown in four columns of two rectangles each that are connected with right pointing arrows. The first rectangle in the upper left is labeled “Volume of N a O H solution.” An arrow points right to a second rectangle labeled “Moles of N a O H added.” A second arrow points right to a third rectangle labeled “Additional moles of N a C H subscript 3 C O subscript 2.” Just beneath the first rectangle in the upper left is a rectangle labeled “Volume of buffer solution.” An arrow points right to another rectangle labeled “Initial moles of C H subscript 3 C O subscript 2 H.” This rectangle points to the same third rectangle, which is labeled “ Additional moles of N a C H subscript 3 C O subscript 2.” An arrow points right to a rectangle labeled “ Unreacted moles of C H subscript 3 C O subscript 2 H.” An arrow points from this rectangle to a rectangle below labeled “[ C H subscript 3 C O subscript 2 H ].” An arrow extends below the “Additional moles of N a C H subscript 3 C O subscript 2” rectangle to a rectangle labeled “[ C H subscript 3 C O subscript 2 ].” This rectangle points right to the rectangle labeled “[ C H subscript 3 C O subscript 2 H ].”](/ocw/mirror/col11830_1.13_complete/m51123/CNX_Chem_14_06_steps2_img.jpg)

