| << Chapter < Page | Chapter >> Page > |
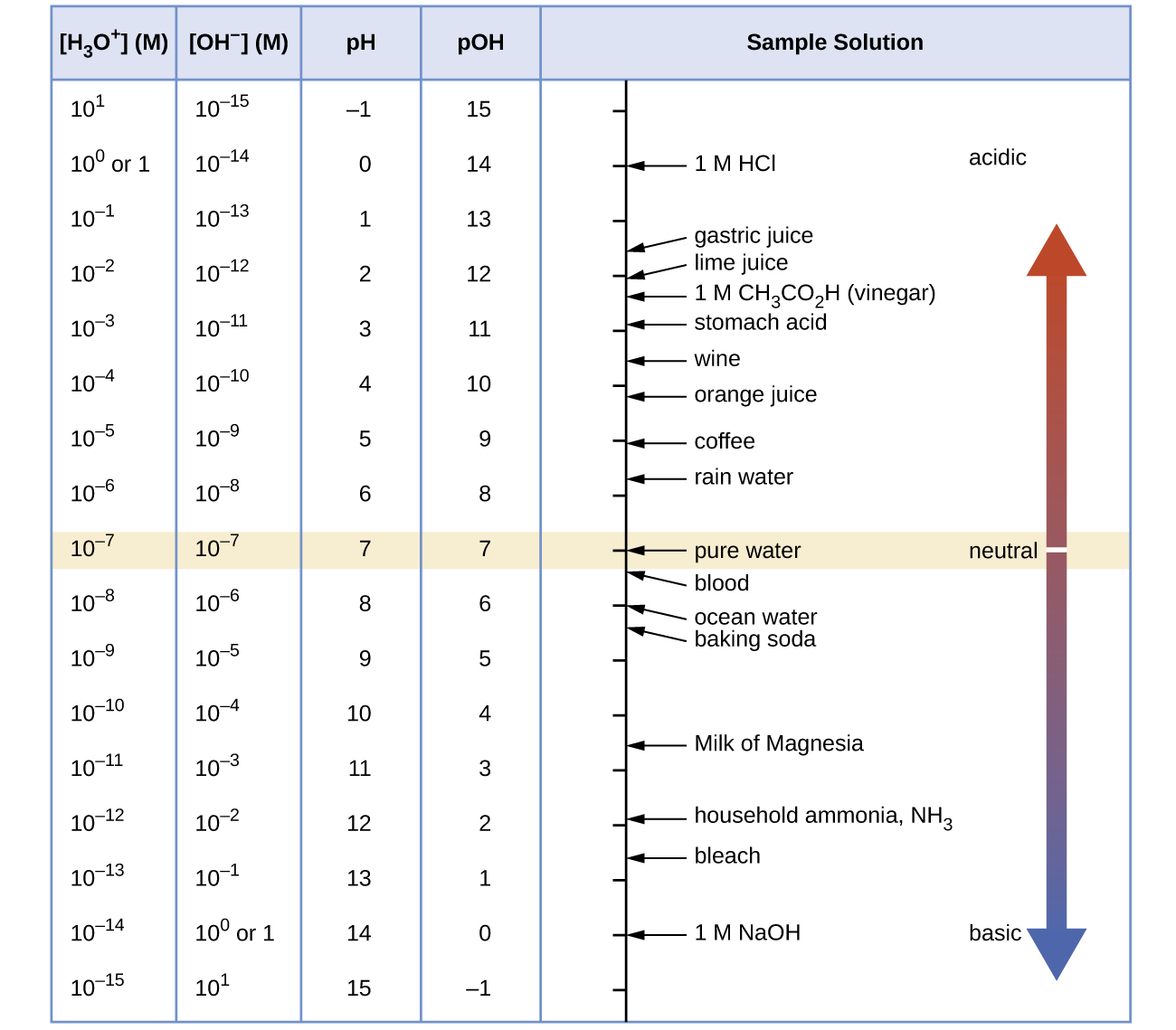
At this temperature, then, neutral solutions exhibit pH = pOH = 6.31, acidic solutions exhibit pH less than 6.31 and pOH greater than 6.31, whereas basic solutions exhibit pH greater than 6.31 and pOH less than 6.31. This distinction can be important when studying certain processes that occur at nonstandard temperatures, such as enzyme reactions in warm-blooded organisms. Unless otherwise noted, references to pH values are presumed to be those at standard temperature (25 °C) ( [link] ).
| Summary of Relations for Acidic, Basic and Neutral Solutions | ||
|---|---|---|
| Classification | Relative Ion Concentrations | pH at 25 °C |
| acidic | [H 3 O + ]>[OH − ] | pH<7 |
| neutral | [H 3 O + ] = [OH − ] | pH = 7 |
| basic | [H 3 O + ]<[OH − ] | pH>7 |
[link] shows the relationships between [H 3 O + ], [OH − ], pH, and pOH, and gives values for these properties at standard temperatures for some common substances.
Air-saturated water has a hydronium ion concentration caused by the dissolved CO 2 of 2.0 10 −6 M , about 20-times larger than that of pure water. Calculate the pH of the solution at 25 °C.
5.70
12 M
Normal rainwater has a pH between 5 and 6 due to the presence of dissolved CO 2 which forms carbonic acid:
Acid rain is rainwater that has a pH of less than 5, due to a variety of nonmetal oxides, including CO 2 , SO 2 , SO 3 , NO, and NO 2 being dissolved in the water and reacting with it to form not only carbonic acid, but sulfuric acid and nitric acid. The formation and subsequent ionization of sulfuric acid are shown here:
Carbon dioxide is naturally present in the atmosphere because we and most other organisms produce it as a waste product of metabolism. Carbon dioxide is also formed when fires release carbon stored in vegetation or when we burn wood or fossil fuels. Sulfur trioxide in the atmosphere is naturally produced by volcanic activity, but it also stems from burning fossil fuels, which have traces of sulfur, and from the process of “roasting” ores of metal sulfides in metal-refining processes. Oxides of nitrogen are formed in internal combustion engines where the high temperatures make it possible for the nitrogen and oxygen in air to chemically combine.
Acid rain is a particular problem in industrial areas where the products of combustion and smelting are released into the air without being stripped of sulfur and nitrogen oxides. In North America and Europe until the 1980s, it was responsible for the destruction of forests and freshwater lakes, when the acidity of the rain actually killed trees, damaged soil, and made lakes uninhabitable for all but the most acid-tolerant species. Acid rain also corrodes statuary and building facades that are made of marble and limestone ( [link] ). Regulations limiting the amount of sulfur and nitrogen oxides that can be released into the atmosphere by industry and automobiles have reduced the severity of acid damage to both natural and manmade environments in North America and Europe. It is now a growing problem in industrial areas of China and India.
For further information on acid rain, visit this website hosted by the US Environmental Protection Agency.


Notification Switch
Would you like to follow the 'Ut austin - principles of chemistry' conversation and receive update notifications?